Research Institute
Department of Pathophysiology of Heart Failure and Therapeutics
Member
| Director | Shigemiki Omiya |
|---|---|
| Laboratory chief | Jumpei Ito |
| Senior Research Scientist | Yuki Kuramoto |
| Research Assistant | Kosuke Nishinaka |
| Postdoctoral fellow | Yohei Tanada |
| Graduate student | Hiroki Nishida Kentaro Mine |
| Research assistant | Azumi Nakamura Atsumi Karasawa Hosoi Mina CAO JINGJING |
| Visiting researcher | Chiaki Nakanishi Kazuto Nunomura Manabu Taneike |
Overall research purpose
In developed countries, heart failure is one of the major causes of death. Novel and effective therapeutics against heart failure need to be developed.
We have reported that autophagy eliminates damaged mitochondria in cardiomyocytes in the pressure-overloaded heart to prevent cardiomyocyte death and maintain cardiac homeostasis (Nat Med. 2007;13. We also found that the inflammatogenic mitochondrial DNA (mtDNA) is degraded by lysosomal deoxyribonuclease II in cardiomyocytes and mtDNA that escapes from autolysosome in autophagy-mediated degradation in cardiomyocytes leads to Toll-like receptor 9-mediated inflammatory responses, cardiomyocyte death and heart failure (Nature. 2012;485) .
Our research involves the application of integrated in vivo physiological studies, molecular, cell biological, imaging and signalling studies using gene-targeting mice to investigate the whole mechanism of the development of heart failure by focusing on each step such as dysregulation of damaged mitochondrial elimination and the induction of inflammation and cardiac cell death etc. We aim to identify novel therapeutic targets and approaches and develop effective therapeutics.
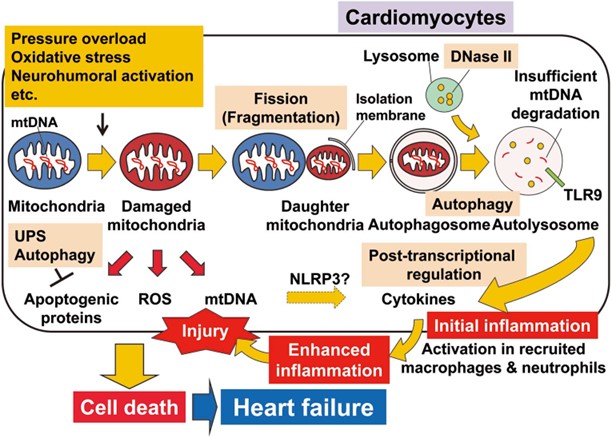
(CircJ. 2017;81.)
Main projects
Sterile inflammation in heart failure:
Cytokine mRNA degradation in cardiomyocytes restrains sterile inflammation in failing hearts (Circulation. 2020;141.). Cardiac fibroblasts produce C-C motif chemokine ligand 2 (Ccl2) that recruits inflammatory immune cells in pressure-overloaded hearts. We investigate the mechanism to regulate the expression of cytokines or chemokines in cardiomyocytes and cardiac fibroblasts during cardiac remodelling.
Mitophagy in heart failure:
Bcl-2-like protein 13 is essential for mitophagy in mammalian cells (Nat. Commun. 2015;6. Cell reports 2019;26). We elucidate the in vivo role of Bcl-2-like protein 13 in heart failure.
Iron homeostasis in failing hearts:
Iron metabolism in heart failure patients is dysregulated (Current Opinion in Cardiology. 2020;35), but it remains unclear whether these changes are pathogenetic and detri-
mental or adaptative and protective for the heart. Ferritin heavy chain, which has ferroxidase activity, is degraded by nuclear receptor coactivator 4 (NCOA4)-mediated autophagic degradation, known as ferritinophagy in pressure-overloaded hearts (J Mol Cell Cardiol. 2009;46. eLife. 2021;10). We investigate the role of iron homeostasis in failing hearts.
Exploration of the new therapeutic targets and development of the new therapeutic strategy:
We examine the mechanism to regulate atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) and develop a new therapy. We also explore a new therapeutic target using induced pluripotent stem cell (iPSC) technology. iPSCs will be generated from a patient with heart failure and those iPSCs will be differentiated into cardiomyocytes (iPSC-CMs) to screen the effect of drugs on the cells.
Please contact our director (omiya.shigemiki@ncvc.go.jp) if you are interested in our research. Scientists from any country are welcome to our group.
Publication
Research for pathophysiology of heart failure
- Murakawa T, Ito J, Rusu MC, Taneike M, Omiya S, Moncayo-Arlandi J, Nakanishi C, Sugihara R, Nishida H, Mine K, Fleck R, Zhang M, Nishida K, Shah AM, Yamaguchi O, Sakata Y, Otsu K. AMPK regulates Bcl2-L-13-mediated mitophagy induction for cardioprotection. Cell Rep. 2024 Dec 24;43(12):115001. PMID: 39580803.
- Sugihara R, Taneike M, Murakawa T, Tamai T, Ueda H, Kitazume-Taneike R, Oka T, Akazawa Y, Nishida H, Mine K, Hioki A, Omi J, Omiya S, Aoki J, Ikeda K, Nishida K, Arita M, Yamaguchi O, Sakata Y, Otsu K. Lysophosphatidylserine induces necrosis in pressure overloaded male mouse hearts via G protein coupled receptor 34. Nat Commun. 2023 Jul 31;14(1):4494. PMID: 37524709.
- Akazawa Y, Taneike M, Ueda H, Kitazume-Taneike R, Murakawa T, Sugihara R, Yorifuji H, Nishida H, Mine K, Hioki A, Omiya S, Nakayama H, Yamaguchi O, Yoshimori T, Sakata Y, Otsu K. Rubicon-regulated beta-1 adrenergic receptor recycling protects the heart from pressure overload. Sci. Rep. 2022;12(41). PMID: 34996972
- Abe H, Tanada Y, Omiya S, Podaru M-N, Murakawa T, Ito J, Shah AM, Conway SJ, Ono M, Otsu K. NF-kB activation in cardiac fibroblasts results in the recruitment of inflammatory
Ly6Chi monocytes in pressure-overloaded hearts. Sci. Signal. 2021;14(704):eabe4932. PMID: 34637330 - Ito J, Omiya S, Rusu M-C, Ueda H, Murakawa T, Tanada Y, Abe H, Nakahara K, Asahi M, Taneike M, Nishida K, Shah AM, Otsu K. Iron derived from autophagy-mediated ferritin degradation induces cardiomyocyte death and heart failure in mice. eLife. 2021;10:e62174:1- 23. PMID: 33526170
- Omiya S, Omori Y, Taneike M, Murakawa T, Ito J, Tanada Y, Nishida K, Yamaguchi O, Satoh T, Shah AM, Akira S, Otsu K. Cytokine mRNA Degradation in Cardiomyocytes Restrains Sterile Inflammation in Pressure-Overloaded Hearts. Circulation. 2020;141(8):667- 677. PMID: 31931613
- Ueda H, Yamaguchi O, Taneike M, Akazawa Y, Wada-Kobayashi H, Sugihara R, Yorifuji H, Nakayama H, Omiya S, Murakawa T, Sakata Y, Otsu K. Administration of a TLR9 Inhibitor Attenuates the Development and Progression of Heart Failure in Mice. JACC: Basic Transl. Sci. 2019;4(3):348-363. PMID: 31312759
- Kitazume-Taneike R, Taneike M, Omiya S, Misaka T, Nishida K, Yamaguchi O, Akira S, Shattock MJ, Sakata S, Otsu K. Ablation of Toll-like receptor 9 attenuates myocardial ischemia/reperfusion injury in mice. Biochem Biophys Res Commun. 2019;515(3), 442-447. PMID: 31160091
- Murakawa T, Okamoto K, Omiya S, Taneike M, Yamaguchi O, Otsu K. A Mammalian Mitophagy Receptor, Bcl2-L-13, Recruits the ULK1 Complex to Induce Mitophagy. Cell reports. 2019;26(2):338-345. PMID: 30625316
- Omiya S, Omori Y, Taneike M, Protti A, Yamaguchi O, Akira S, Shah AM, Nishida K, Otsu K. Toll-like receptor 9 prevents cardiac rupture after myocardial infarction in mice independently of inflammation. Am J Physiol Heart Circ Physiol. 2016;311(6):H1485-H1497. PMID: 27769998
- Murakawa T, Yamaguchi O, Hashimoto A, Hikoso S, Takeda T, Oka T, Yasui H, Ueda H, Akazawa Y, Nakayama H, Taneike M, Misaka T, Omiya S, Shah AM, Yamamoto A, Nishida K, Ohsumi Y, Okamoto K, Sakata Y, Otsu K. Bcl-2-like protein 13 is a mammalian Atg32 homologue that mediates mitophagy and mitochondrial fragmentation. Nat Commun. 2015;6:7527 . PMID: 26146385
- Oka T, Hikoso S, Yamaguchi O, Taneike M, Takeda T, Tamai T, Oyabu T, Murakawa T, Nakayama H, Nishida K, Akira S, Yamamoto A, Komuro I, Otsu K. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature. 2012;10;485(7397):251-5. PMID: 22535248
- Hikoso S, Yamaguchi O, Nakano Y, Takeda T, Omiya S, Mizote I, Taneike M, Oka T, Tamai T, Oyabu J, Uno Y, Matsumura Y, Nishida K, Suzuki K, Kogo M, Hori M, Otsu K. The IkB kinase b/nuclear factor kB signaling pathway protects the heart from hemodynamic stress mediated by the regulation of manganese superoxide dismutase expression. Circ Res. 2009;105(1):70-9. PMID: 19478205
- Omiya S, Hikoso S, Imanishi Y, Saito A, Yamaguchi O, Takeda T, Mizote I, Oka T, Taneike M, Nakano Y, Matsumura Y, Nishida K, Sawa Y, Hori M, Otsu K. Downregulation of ferritin heavy chain increases labile iron pool, oxidative stress and cell death in cardiomyocytes. J Mol Cell Cardiol. 2009;46(1):59-66. PMID: 18992754
- Watanabe T, Takeda T, Omiya S (Co-first author), Hikoso S, Yamaguchi O, Nakano Y, Higuchi Y, Nakai A, Abe Y, Aki-Jin Y, Taniike M, Mizote I, Matsumura Y, Shimizu T, Nishida K, Imai K, Hori M, Shirasawa T, Otsu K. Reduction in hemoglobin-oxygen affinity results in the improvement of exercise capacity in mice with chronic heart failure. J Am Coll Cardiol. 2008;52(9):779-86. PMID: 18718428
- Nakai A, Yamaguchi O (Co-first author), Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, Nishida K, Hori M, Mizushima N, and Otsu K. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nature Med. 2007;13(5):619-624. PMID: 17450150
- Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434(7033):652-8. PMID: 15800626
- Yamaguchi O, Higuchi Y, Hirotani S, Kashiwase K, Nakayama H, Shungo H, Takeda T, Watanabe T, Asahi M, Taniike M, Matsumura Y, Tsujimoto I, Hongo K, Kusakari Y, Kurihara S, Nishida K, Ichijo H, Hori M, Otsu K. Targeted deletion of apoptosis signal-regulating kinase 1 attenuates left ventricular remodeling. Proc Natl Acad Sci U S A. 2003; 100(26):15883-8. PMID: 14665690
last updated : 2025/04/11
