Division of Cerebrovascular Medicine and Neurology
Clinical Team: Neurology (Brain medicine B)
Our research interests have focused on the radiological, ultrasonological and pathological changes that occur in stroke and dementia and how the alterations impact on brain health during old age. We target a wide range of stroke related diseases and conditions such as acute ischemic/hemorrhagic stroke, vascular dementia, poststroke epilepsy, and vascular parkinsonism. We are currently involved in translational research projects for stroke or mild cognitive impairment including clinical trials using new endovascular device for acute stroke, a vasoactive drug for mild cognitive impairment, and peptide hormone adrenomedullin for ischemic stroke. We have also used brain autopsy materials and experimental rodent models to publish widely on these issues in collaboration with our Research Institute. Our department members have his/her own expertise to promote the following projects:
- Development of pre-hospital stroke scale to identify acute ischemic stroke caused by large vessel occlusion.
- Development of a quantitative evaluation method for the cerebral collateral circulation
- A study to establish an algorithm for predicting recovery after stroke
- Research on etiology of stroke (especially cerebral hemorrhage)
- Association between juvenile stroke and the Moyamoya-related RNF213 p.R4810K polymorphism
- Ultrasound diagnosis for the vulnerability of carotid arteries plaques
- PROgnosis of Post Stroke Epilepsy (PROPOSE) Study
- Effects of genetic polymorphisms on the pharmacokinetics of anticoagulants study
- Research on the pathogenesis of hereditary vascular dementia CADASIL and CARASIL
- Vascular factors in Alzheimer's disease
- Cilostazol for Prevention of Conversion from MCI to Dementia (COMCID study)
- Development of rehabilitation program using virtual reality technology
- Finger tapping for evaluating early cognitive impairment
- The therapeutic potential of adrenomedulin (AM) in ischemic stroke
- Generation of animal models of sporadic vascular dementia
1. Development of pre-hospital stroke scale to identify acute ischemic stroke caused by large vessel occlusion.
Patients with acute ischemic stroke caused by large vessel occlusion (LVO, occlusion of internal artery or proximal middle cerebral) present severe neurological symptoms. Furthermore, LVO is hardly recanalized by intravenous thrombolysis. Recently, efficacy of endovascular therapy in patients with acute ischemic stroke with LVO has been clarified. However, the efficacy of endovascular therapy depends on the time from symptom onset to initiation of this therapy. These patients should be transferred to comprehensive stroke center and treated with endovascular therapy immediately.
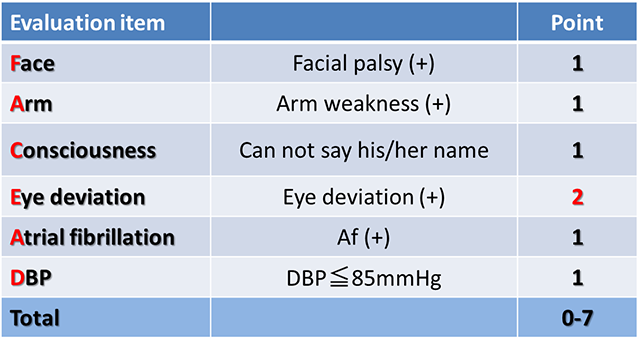
We have designed a simple prehospital stroke scale, FACE2-AD scale, by which emergency medical personnel can easily identify patients with acute ischemic stroke due to LVO. A validation study using this scale by paramedics is ongoing. We are planning to construct regional stroke care systems using the FACE2-AD scale (Okuno Y, et al. Transl Stroke Res 2020).
Transfer and direct transport
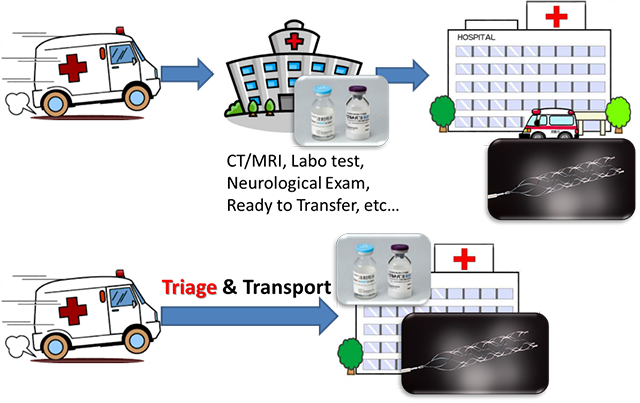
Appropriate triage of LVO eligible patients and direct transport to the endovascular-capable stroke center can shorten the time from onset to treatment.
FACE2-AD (FACE to Acute Delivery score)
2. 2.Development of a quantitative evaluation method for the cerebral collateral circulation
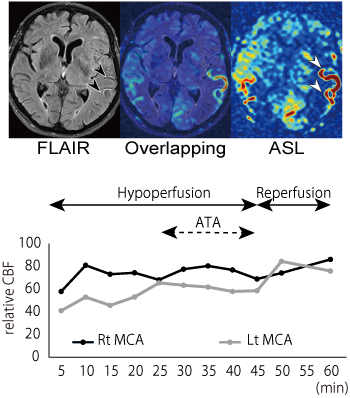
The aim of this collaborative work with radiology and bio-medical imaging department is to establish a new quantitative evaluation method for the cerebral collateral circulation after acute ischemic stroke by using non-invasive three-dimension arterial spin labeling perfusion MRI (3D-ASL). We have reported that 3D-ASL during intravenous thrombolysis can evaluate the leptomeningeal collateral flow and predict early reperfusion after thrombolysis (Figure 1; Okazaki et al, Stroke 2016). Our recent studies have also revealed that focal hyperperfusion after reperfusion therapy detected by 3D-ASL is well associated with hemorrhagic transformation.
According to these findings, we are now developing a new algorithm for selecting candidates for reperfusion therapy and a new quantitative evaluation method for the cerebral collateral circulation.
Figure 1. Repeated ASL perfusion images during intravenous thrombolysis.
Upper image: ASL shows arterial transit artifacts (ATA) in the hypoperfused area (white arrow heads). FLAIR shows hyperintense vessel sign in the corresponding site of ATA (black arrow heads). Lower image: relative cerebral blood flow (CBF) changes during thrombolysis
3. A study to establish an algorithm for predicting recovery after stroke
The aim of this study is to establish a new algorithm for predicting recovery after stroke in combination of neurophysiological techniques and MRI in the acute phase. By insertion of this algorithm into the inclusion criteria of future clinical trials, we aim to establish effective neuroprotective drugs.
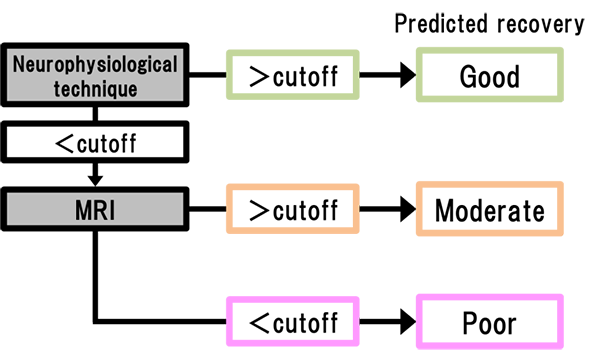
Figure. Proposed algorithm for prognosis prediction for stroke.
4. Research on etiology of stroke (especially cerebral hemorrhage)
A collaborative work with Osaka University Graduate School of Dentistry (Prof. Nakano) showed that Streptococcus mutans is a strong risk factor of cerebral hemorrhage(Tonomura S, et al. Sci Rep 2016; Ihara M, et al. Future Neurol 2018). Although reduced in incidence due to better control of hypertension, cerebral hemorrhage is still prevalent in Japan compared to western countries, and we hope this novel finding will lead to development of new treatment of cerebral hemorrhage. Recently, we reported that this bacteria is associated with non-alcoholic steatohepatitis (NASH) (Tonomura S, et al. BMJ Open Gastroenterol 2019), suggesting the wide-range role of Streptococcus mutans with human diseases.
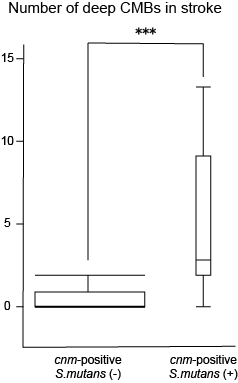
Figure. Stroke patients with cnm-positive S. mutans showed a significantly higher number of deep microbleeds.
5. Association between juvenile stroke and the Moyamoya-related RNF213 p.R4810K polymorphism
Stroke in young adults accounts for about 10% of all stroke. Although some genetic predispositions have been previously reported, no susceptibility gene for juvenile stroke has been identified to date. We hypothesize that RNF213 p.R4810K polymorphism, the susceptibility gene for moyamoya disease, is also associated with the development of juvenile stroke in Asia (Figure 2). This is a collaborative study with the Department of Health and Environmental Sciences in Kyoto Univ. Graduate School of Medicine (Okazaki S, et al. Circulation 2019; Kamimura T, et al. Stroke 2019).
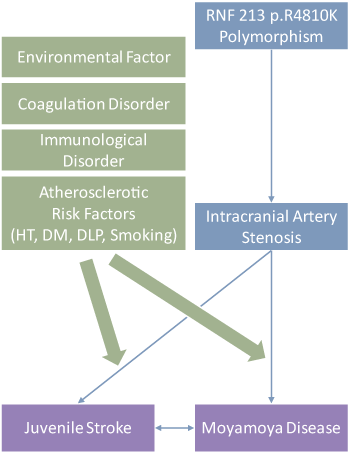
Figure 2. Hypothesis of the development of juvenile stroke.
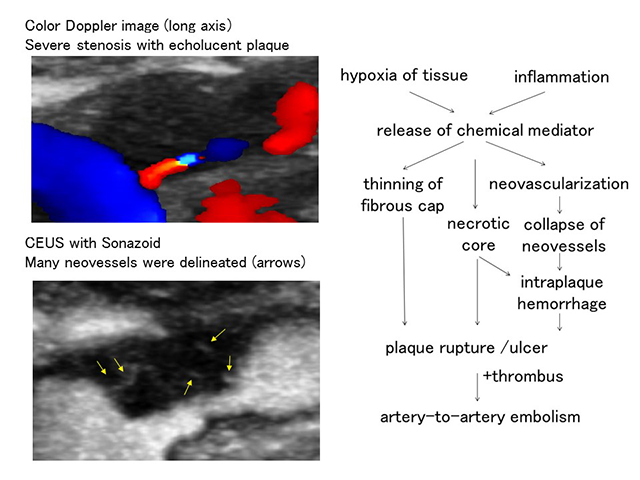
6. Ultrasound diagnosis for the vulnerability of carotid arteries plaques
Atherosclerotic plaques contain necrotic core, which may result in plaque rupture and cause artery-to-artery embolism. We evaluate plaque vulnerability using contrast enhanced ultrasound (CEUS). CEUS can delineate intraplaque neovessels, and contrast effect of symptomatic plaques was significantly greater than that of asymptomatic plaques (Saito et al. Stroke 2014). CEUS can also detect small ulcers which could not be delineated using color Doppler mode.
We aim for a better qualitative evaluation of carotid plaques.
7. PROgnosis of Post Stroke Epilepsy (PROPOSE) Study
Post stroke epilepsy (PSE) is a clinical problem for stroke survivors. The incidence rate of PSE was about 10 %. Previous reports showed that the occurrence of PSE led to poor prognosis and increased mortality.
Moreover, PSE has the high recurrence rate. Our previous study showed that PSE recurred in 30% patients within 360 days (Tanaka et al. PLoS One 2015). PSE patients are frightened by the recurrence in their daily lives; therefore, PSE may worsen the recovery and quality of life in stroke survivors (Tanaka T and Ihara M. Neurochem Int 2017). As there are still many questions about PSE, we conducted multicentre prospective cohort study of PSE, which was supported by AMED. Subsequently, 511 PSE patients were registered in the cohort, which is now subject to analyses to know the clinical and neuroradiological characteristics of Japanese PSE patients and drug effects for prevention of PSE recurrence. In addition, we hold hospital meeting of PSE every week and EEG reading conference once a month with several institutes including Kyoto University. Moreover, we are developing new diagnosis methods and algorithm of PSE using SPECT imaging.
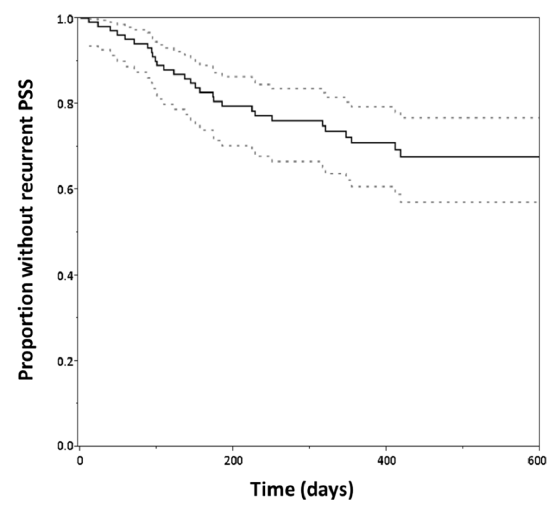
Figure.
Kaplan-Meier curve for proportion without recurrent PSE. After a median follow-up of 362 days, we identified recurrent PSE in 31 patients(29.8%). Dotted lines indicate the confidence interval.

8. Effects of genetic polymorphisms on the pharmacokinetics of anticoagulants study
In recent days, it became possible to use DOAC as anticoagulants without requiring dose adjustment. However, there are individual differences in the blood concentration of anticoagulants, which may affect the drug efficacy, due to genetic polymorphisms.
In cooperation with our pharmaceutical and clinical laboratory examination departments, we are evaluating the concentration and genetic polymorphisms by using the triple quadrupole liquid chromatograph mass spectrometer (Shimadzu, LCMS-8030) and the gene analysis system (Shimadzu, GTS-7000).

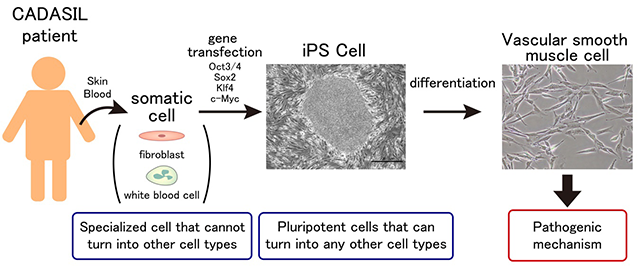
9. Research on the pathogenesis of hereditary vascular dementia CADASIL and CARASIL
CADASIL (cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy) and CARASIL (cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy) are hereditary small vessel disease caused by mutations in NOTCH3 and HTRA1, respectively (Yamamoto Y and Ihara M. Neurochem Int 2017). Although the causative genes have been identified, their molecular pathogenesis is still unclear. We are investigating the pathogenesis of hereditary vascular dementia using animal models of CADASIL and CARASIL as well as induced pluripotent stem cells (iPSCs) from CADASIL patients. Currently we are also working on the establishment of CADASIL patient database. We aim to expand our research further to establish Japanese diagnostic criteria of CADASIL and develop new treatment in the future.
10. Vascular factors in Alzheimer's disease
The elderly people often have multiple comorbidities. The most common cause of dementia, Alzheimer's disease, is no exception; it is accompanied by lifestyle-related disorders such as hypertension and diabetes mellitus. We therefore try to elucidate the underlying mechanism by which vascular disease accelerates neurodegenerative processes and to find treatment of Alzheimer's disease by targeting vascular diseases (Saito S, et al. Front Neurol 2019).
Studies of transgenic mice expressing tau gene mutations in a familial form of tauopathy (FTDP-17) implicate pathological tau in mechanisms of neurodegenerative dementia. In case of AD, however, the relationship between Aβ and tau has not been clearly elucidated. We are investigating the mechanism from both point of view; Aβ and tau.
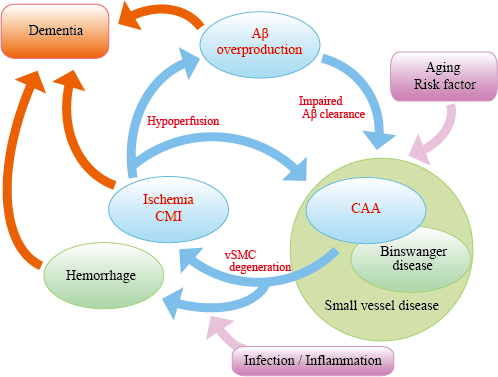
Figure "AD malignant cycle". Abeta overproduction impairs Abeta elimination leading to vascular smooth muscle cells (vSMC) degeneration,cerebral ischemia, and microinfarcts. Ischemia also induces Abeta overproduction. Such vicious circle consists of core pathology in sporadic AD.Note that cessation of Abeta overproduction is not sufficient to sever the cycle.
11. Cilostazol for Prevention of Conversion from MCI to Dementia (COMCID study)

Epidemiological, clinicopathological and animal studies show that vascular disease in various forms contributes to cognitive decline. Increasing age is the strongest risk for dementia irrespective of whether it results from a vascular etiology or neurodegenerative disease processes such as in Alzheimer's disease (AD). AD and vascular cognitive impairment, the two most common causes of dementia, represent two extremes of a spectrum of disorders; however, a number of entities, which possess varying degrees of neurodegenerative and vascular pathologies, occur in between. The pure forms of the disorders are preferred for convenience to label, treat or manage but conditions within the spectrum are the norm rather than the exception as dementia advances. Therefore, combinatorial therapy directed at both vascular and neurodegenerative aspects of dementia is a promising approach for the treatment of dementia in the elderly.
- ClinicalTrials.gov:
https://clinicaltrials.gov/ct2/show/NCT02491268?term=comcid&rank=1 - Alzheimer's & Dementia: Translational Research & Clinical Interventions:
http://www.trci.alzdem.com/article/S2352-8737%2816%2930040-3/fulltext
12. Development of rehabilitation program using virtual reality technology
It is clear that rehabilitation will lead to improvement of motor function, but it is difficult to continue monotonous rehabilitation. Due to progress of virtual reality technology, applications of three dimensional space images have been studied in various fields; however, there are few studies in the stroke rehabilitation field. We cooperate with Osaka University and Medi VR Inc. to create novel virtual reality program for rehabilitation after stroke.

13. Finger tapping for evaluating early cognitive impairment
With continuous increase of elderly people in Japan, dementia has become a major social issue. It is important to detect cognitive impairment at early stage because relevant medication and life support may somewhat prevent the disease progression. At present, however, Minimental State Examination (MMSE), a well-known screening test for simplicity and utility, is insufficient for evaluating executive function including motor skill function. Thus, we are conducting a finger tapping study (Fig 2) using "UB-1"magnetic detection system produced by HITACHI Ltd (Fig 1).
Fig 3 and 4 show right finger-tapping data while subjects tap both fingers at the same time. Both examinees achieved normal score in MMSE but Frontal Assessment Battery (FAB, which is known to be related with executive function) was normal in Fig 3 but low in Fig 4.
Further study is being planned to establish early stage of dementia, especially focusing on stroke-related one.
Fig 1 |
Fig 2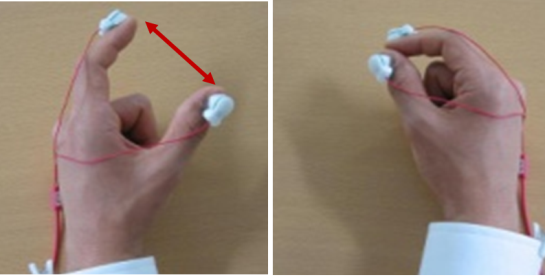 |
Fig 3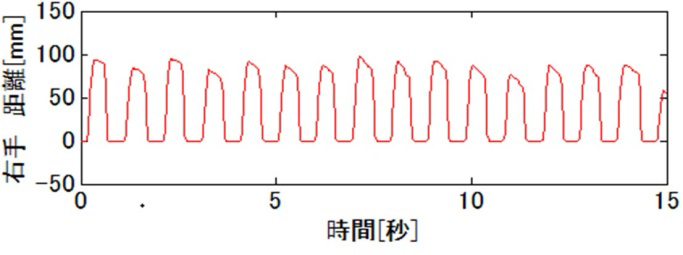 |
Fig 4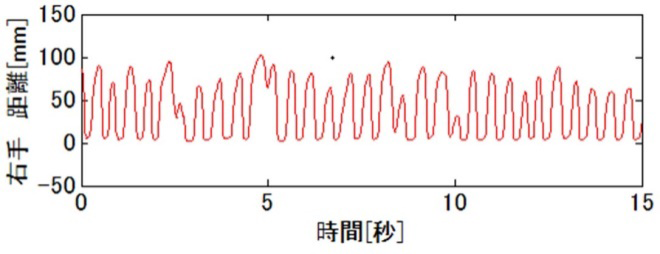 |
14. The therapeutic potential of adrenomedulin in ischemic stroke
Although the mortality rate of stroke has decreased due to the progress of acute therapies of infarction such as recombinant tissue plasminogen activator and endovascular thrombectomy, the next stage of therapy is being desired for further improvement in stroke mortality and morbidity.
Adrenomedullin (AM) has a variety of effects on the vasculature that include vasodilation, regulation of permeability, inhibition of endothelial cell apoptosis and oxidative stress, regulation of smooth muscle cell proliferation, and promotion of angiogenesis. We previously reported that after bilateral common carotid artery stenosis (BCAS), mice overexpressing circulating AM showed significantly faster cerebral perfusion recovery due to substantial growth of the capillaries, the circle of Willis, and the leptomeningeal anastomoses and reduced oxidative damage in vascular endothelial cells compared with wild-type mice(Maki T, et al. Stroke 2011 ). A investigator-initiated translational research will be started in 2020, funded by AMED.
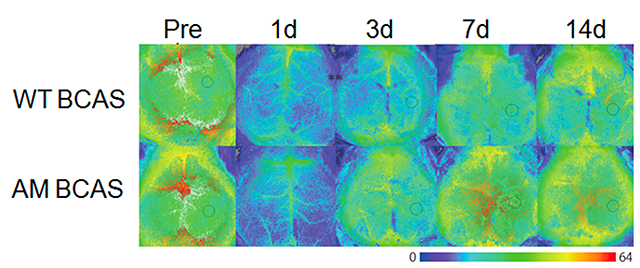
Figure.
Images showing temporal changes of CBF in wild-type (WT) and AM-Tg mice after BCAS. On Days 1 and 3 post-BCAS, there was a slight, but not significant, increase in CBF in mice overexpressing circulating AM BCAS compared with WT BCAS. On Day 7 post-BCAS, AM BCAS showed significantly faster cerebral perfusion recovery: cerebral perfusion was significantly higher in AM BCAS compared with WT BCAS.
15. Generation of animal models of sporadic vascular dementia
We are developing animal models of sporadic vascular dementia using mice and rats which can be applied to drug development for human vascular dementia. Recently, we have established a mouse model of vascular dementia named ACAS (asymmetric common carotid artery surgery) model (Hattori Y, et al. J Neurosci 2015). For this publication, our staff Dr. Yorito Hattori won the Kusano prize in 2016. Our review paper about cerebral small vessel disease was published in Stroke and one of the figures was printed in cover page (Ihara M and Yamamoto Y. Stroke 2016) (see figure below).
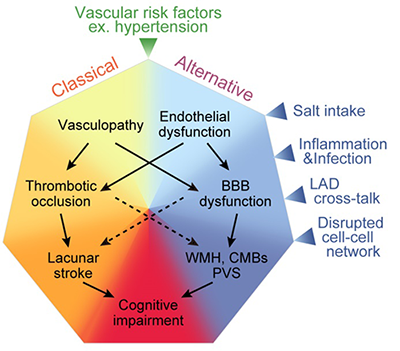
Figure. Mechanism of cerebral small vessel disease. In addition to the old pathway consisting of arteriolosclerosis -> thrombosis formation -> cerebral infarction -> dementia, a lot of 'unknown' factors such as salt toxicity, inflammation/infection, large and small artery crosstalk, and disrupted cell-cell interaction in the blood-brain barrier seem to contribute to the pathogenesis of cerebral small artery disease.
Selected publications in recent 5 years
- Okuno Y, Yamagami H, Kataoka H, Tahara Y, Tonomura S, Tokunaga H, Imahori T, Matsui D, Kobayashi M, Imamura H, Sakai N, Takahashi JC, Toyoda K, Nagatsuka K, Ihara M. Field Assessment of Critical Stroke by Emergency Services for Acute Delivery to a Comprehensive Stroke Center: FACE2AD. Transl Stroke Res, in press
- Yoshimoto T, Inoue M, Yamagami H, Fujita K, Tanaka K, Ando D, Sonoda K, Kamogawa N, Koga M, Ihara M, Toyoda K. Use of Diffusion-Weighted Imaging-Alberta Stroke Program Early Computed Tomography Score (DWI-ASPECTS) and Ischemic Core Volume to Determine the Malignant Profile in Acute Stroke. J Am Heart Assoc 2019;8(22):e012558. doi: 10.1161/JAHA.119.012558.
- Tonomura S, Naka S, Hara T, Mori K, Tanaka N, Sumida Y, Kanemasa K, Takahashi N, Nomura R, Ihara M, Nakano K. The relationship between Streptococcus mutans harboring Cnm and PA antigens and nonalcoholic steatohepatitis. BMJ Open Gastroenterol 2019;6(1):e000329. doi: 10.1136/bmjgast-2019-000329.
- Kalaria RN, Hase Y, Ihara M. The rise and rise of cerebral small vessel disease: implications for vascular cognitive impairment and dementia. Future Neurol 2019; FNL11. doi: 10.2217/fnl-2019-0004
- Fujita K, Tanaka K, Yamagami H, Ide T, Ishiyama H, Sonoda K, Satow T, Takahashi JC, Ihara M, Koga M, Yokota T, Toyoda K. Detrimental effect of chronic hypertension on leptomeningeal collateral flow in acute ischemic stroke. Stroke 2019;50(7):1751-1757. doi: 10.1161/STROKEAHA.119.025142.
- Nakaoku Y, Saito S, Yamamoto Y, Maki T, Takahashi R, Ihara M. The dipeptidyl peptidase-4 inhibitor linagliptin ameliorates high-fat induced cognitive decline in tauopathy model mice. Int J Mol Sci 2019; 20(10). pii: E2539. doi:10.3390/ijms20102539
- Wada S, Koga M, Makita N, Nakamura Y, Miwa K, Ide T, Yi K, Mizoguchi T, Yamaguchi Y, Ihara M, Toyoda K. Detection of Stenosis Progression in Intracranial Vertebral Artery Dissection Using Carotid Ultrasonography. J Stroke Cerebrovasc Dis 2019. pii: S1052-3057(19)30214-9. doi: 10.1016/j.jstrokecerebrovasdis.2019.04.033.
- Tanaka M, Saito S, Inoue T, Satoh-Asahara N, Ihara M. Novel therapeutic potentials of taxifolin for amyloid-β-associated neurodegenerative diseases and other diseases: recent advances and future perspectives. Int J Mol Sci 2019; 20(9): pii: E2139. https://doi.org/10.3390/ijms20092139
- Inoue T, Saito S, Tanaka M, Yamakage H, Kusakabe T, Shimatsu A, Ihara M, Satoh-Asahara N. Pleiotropic neuroprotective effects of taxifolin in cerebral amyloid angiopathy. Proc Natl Acad Sci USA 2019;116(20):10031-10038. https://doi.org/10.1073/pnas.1901659116
- Saito S, Yamamoto Y, Ihara M. Development of a multicomponent intervention to prevent Alzheimer's disease. Front Neurol 2019;10:490. doi: 10.3389/fneur.2019.00490
- Tanaka T, Yamagami H, Ihara M, Miyata T, Miyata S, Hamasaki T, Amano S, Fukuma K, Yamamoto H, Nakagawara J, Furui E, Uchiyama S, Hyun B, Yamamoto Y, Manabe Y, Ito Y, Fukunaga R, Abumiya T, Yasaksaa M, Kitagawa K, Toyoda K, Nagatsuka K. Association of CYP2C19 Polymorphisms With Clopidogrel Reactivity and Clinical Outcomes in Chronic Ischemic Stroke. Circ J 2019;83(6):1385-1393. doi: 10.1253/circj.CJ-18-1386.
- Ikenouchi H, Washida K, Yoshimoto T, Fukuma K, Saito S, Inoue Y, Matsuda H, Ihara M. Balloon-like mobile plaque in the innominate artery: ultrasonographic and pathological perspectives of repetitive embolism. J Stroke Cerebrovasc Dis 2019 pii: S1052-3057(19)30180-6. doi: 10.1016/j.jstrokecerebrovasdis.2019.04.015.
- Kamimura T, Okazaki S, Morimoto T, Kobayashi H, Harada K, Tomita T, Higashiyama A, Yoshimoto T, Takahashi JC, Nakagawara J, Koga M, Toyoda K, Maruyama H, Koizumi A, Ihara M. The prevalence of RNF213 p.R4810K variant in early-onset stroke with intracranial arterial stenosis. Stroke 2019;50(6):1561-1563 doi: 10.1161/STROKEAHA.118.024712
- Ihara M. A neurovascular approach to clearing β-amyloid from the brain. Vas-Cog J 2019;5(1):4-11.
- Tomari S, Tanaka T, Matsubara S, Fukuma K, Ihara M, Nagatsuka K, Toyoda K. Risk factors for nonconvulsive status epilepticus after stroke. Eur Neurol 2019;80(5-6):256-260. doi: 10.1159/000496512.
- Okazaki S, Morimoto T, Kamatani Y, Kamimura T, Kobayashi H, Harada KH, Tomita T, Higashiyama A, Takahashi J, Nakagawara J, Koga M, Toyoda K, Washida K, Saito S, Takahashi A, Hirata M, Matsuda K, Mochizuki H, Chong M, Paré G, O'Donnell M, Ago T, Hata J, Ninomiya T, Dichgans M, Debette S, Kubo M, Koizumi A, Ihara M. Moyamoya disease susceptibility variant RNF213 p.R4810K increases the risk of ischemic stroke due to large artery atherosclerosis. Circulation 2019;139(2):295-298. doi: 10.1161/CIRCULATIONAHA.118.038439
- Takahashi M, Yamamuro K, Yasuno F, Matsuoka K, Kitamura S, Yoshikawa H, Yamamoto A, Iida H, Fukuda T, Ihara M, Nagatsuka K, Kishimoto T. Early detection of brain amyloid β deposits in the patient at the preliminary stage of chronic traumatic encephalopathy. Psychogeriatrics in press doi: 10.1111/psyg.12383.
- Takahashi Y, Saito S, Yamamoto Y, Uehara T, Yokota C, Sakai Go, Nishida N, Takahashi R, Kalaria RN, Toyoda K, Nagatsuka K, Ihara M. Visually-rated medial temporal lobe atrophy with lower educational history as a quick indicator of amnestic cognitive impairment after stroke. J Alzheimers Dis 2019;67(2):621-629. doi: 10.3233/JAD-180976.
- Fujita K, Sonoda K, Miyata T, Ihara M, Toyoda K, Koga M. Early identification of protein S K196E mutation in a patient with cerebral venous thrombosis: a case report. J Stroke Cerebrovasc Dis 2019;28(1):232-233. doi: 10.1016/j.jstrokecerebrovasdis.2018.09.040.
- Nakaoku Y, Oishi N, Hase Y, Hase M, Saito S, Mitsueda T, Matsui M, Toyoda K, Nagatsuka K, Kalaria RN, Fukuyama H, Ihara M, Takahashi R. Montreal cognitive assessment score correlates with regional cerebral blood flow in post-stroke patients. Clin Neurol Neurosurg 2018; 174: 68-74. doi: 10.1016/j.clineuro.2018.09.004.
- Ihara M, Tonomura S, Yamamoto Y, Saito S. Collagen-binding Streptococcus mutans tied to cerebral microbleeds and hemorrhagic stroke. Future Neurol 2018;13(4): 219-224. https://doi: 10.2217/fnl-2018-0016
- Akinyemi R, Owolabi M, Damasceno A, Ihara M, Ogunniyi A, Dotchin C, Paddick SM, Ogeng'o J, Walker R, Kalaria RN. Stroke, cerebrovascular diseases and vascular cognitive impairment in Africa. Brain Res Bull 2019;145:97-108. doi: 10.1016/j.brainresbull.2018.05.018.
- Ishiyama H, Okazaki S, Saito K, Yamagami H, Ihara M. Rolling stones sign as hard-and-fast evidence of calcified cerebral emboli. Neurology 2018;91:41-43. doi: 10.1212/WNL.0000000000005731.
- Kinoshita N, Saito K, Yamaguchi Y, Abe S, Wada S, Tanaka T, Kajimoto K, Yamagami H, Maruyama H, Toyoda K, Ihara M, Nagatsuka K. Flip flop phenomenon: swallowing-induced arterial displacement as an indicator of carotid artery disease. Cerebrovasc Dis 2018;45(5-6):258-262. doi.org/10.1159/000490180
- Uemura M, Ihara M, Maki T, Nakagomi T, Kaji S, Uemura K, Matsuyama T, Kalaria RN, Kinoshita A, Takahashi R. Pericyte-derived bone morphogenetic protein 4 underlies white matter damage after chronic hypoperfusion. Brain Pathol 2018;28(4):521-535. doi: 10.1111/bpa.12523.
- Wada S, Yoshimura S, Inoue M, Matsuki T, Arihiro S, Koga M, Kitazono T, Makino H, Hosoda K, Ihara M, Toyoda K. Outcome prediction in acute stroke patients by continuous glucose monitoring. J Am Heart Assoc 2018;7(8). pii: e008744. doi: 10.1161/JAHA.118.008744.
- Toyoda K, Koga M, Yamagami H, Yokota C, Sato S, Inoue M, Tanaka T, Endo K, Fujinami J, Ihara M, Nagatsuka K, Minematsu K. Seasonal variations in neurological severity and outcomes of ischemic stroke- 5-year single-center observational study. Circ J. 2018;82(5):1443-1450. doi: 10.1253/circj.CJ-17-1310.
- Morimoto T, Enmi J, Hattori Y, Iguchi S, Saito S, Harada K, Okuda H, Takagi Y, Youssefian S, Iida H, Miyamoto S, Ihara M, Kobayashi H, Koizumi A. Dysregulation of RNF213 promotes cerebral hypoperfusion. Sci Rep 2018;8:3607. doi: 10.1038/s41598-018-22064-8.
- Ihara M, Washida K. Linking atrial fibrillation with Alzheimer's disease: epidemiological, pathological, and mechanistic evidence. J Alzheimers Dis 2018;62(1):61-72. doi: 10.3233/JAD-170970.
- Hase Y, Horsburgh K, Ihara M, Kalaria RN. White matter degeneration in vascular and other ageing-related dementias. J Neurochem 2018;144(5):617-633. doi: 10.1111/jnc.14271.
- Duncombe J, Kitamura A, Hase Y, Ihara M, Kalaria RN, Horsburgh K. Chronic cerebral hypoperfusion: a key mechanism leading to vascular cognitive impairment and dementia. Closing the translational gap between rodent models and human vascular cognitive impairment and dementia. Clin Sci 2017;131(19):2451-2468. doi: 10.1042/CS20160727.
- Tomari S, Tanaka T, Ihara M, Matsuki T, Fukuma K, Matsubara S, Nagatsuka K, Toyoda K. Risk factors for post-stroke seizure recurrence after the first episode. Seizure 2017;52:22-26. doi: 10.1016/j.seizure.2017.09.007
- Kinoshita N, Saito K, Tanaka T, Kajimoto K, Yamagami H, Morita Y, Ihara M, Nagatsuka K. Flip flop phenomenon: swallowing-induced displacement of the carotid artery as a risk of stroke. Neurology 2017;89(15):1643-1644. 10.1212/WNL.0000000000004492
- Okazaki S, Yamagami H, Yoshimoto T, Morita Y, Yamamoto H, Toyoda K, Ihara M. Cerebral hyperperfusion on arterial spin labeling MRI after reperfusion therapy is related to hemorrhagic transformation. J Cereb Blood Flow Metab 2017;37(9):3087-3090. 10.1177/0271678X17718099
- Offner H, Ihara M, Schäbitz WR, Wong PT. Stroke and other cerebrovascular diseases. Neurochem Int 2017;107:1-3. doi: 10.1016/j.neuint.2017.04.003.
- Saito S, Yamamoto Y, Maki T, Hattori Y, Ito H, Mizuno K, Harada-Shiba M, Kalaria RN, Fukushima M, Takahashi R, Ihara M. Taxifolin inhibits amyloid-β oligomer formation and fully restores vascular integrity and memory in cerebral amyloid angiopathy. Acta Neuropathol Commun 2017;5:26. 10.1186/s40478-017-0429-5
- Tanaka T, Ihara M. Post-stroke epilepsy. Neurochem Int 2017;107:219-228. doi: 10.1016/j.neuint.2017.02.002.
- Kalaria RN, Ihara M. Medial temporal lobe atrophy is the norm in cerebrovascular dementias. Eur J Neurol 2017;24(4):539-540.doi: 10.1111/ene.13243
- Hainsworth AH, Allan S, Boltze J, Cunningham C, Farris C, Head E, Ihara M, Isaacs JD, Kalaria RN, Lesnik-Oberstein SAJ, Moss M, Nitzsche B, Rosenberg GA, Rutte JW, Salkovic-Petrisic M, Troen AM. Translational Models for Vascular Cognitive Impairment. A Review Including Larger Species. BMC Med 2017;15:16. doi: 10.1186/s12916-017-0793-9.
- Yamamoto Y, Ihara M. Disruption of transforming growth factor-β superfamily signaling: A shared mechanism underlying hereditary cerebral small vessel disease. Neurochem Int 2017;107:211-218. doi: 10.1016/j.neuint.2016.12.003.
- Saito S, Kojima S, Oishi N, Kakuta R, Maki T, Yasuno F, Nagatsuka K, Yamamoto H, Fukuyama H, Fukushima M, Ihara M. A multicenter, randomized, placebo-controlled trial for cilostazol in patients with MCI: the COMCID study protocol. Alzheimers Dement (N Y) 2016; 2: 250-257. doi: 10.1016/j.trci.2016.10.001.
- Hattori Y, Enmi J, Iguchi S, Saito S, Yamamoto Y, Nagatsuka K, Iida H, Ihara M. Substantial reduction of parenchymal cerebral blood flow in mice with bilateral common carotid artery stenosis. Sci Rep 2016; 6: 32179.
- Fukuma K, Ihara M, Miyashita K, Motoyama R, Tanaka T, Katsufumi K, Ikeda A, Nagatsuka K. Right parietal source in Mahjong-induced seizure: a system epilepsy of focal origin. Clin Case Rep 2016;4(10):948-951.
- Doijiri R, Uno H, Miyashita K, Ihara M, Naritomi H, Nagatsuka K. How commonly is stroke found in patients with isolated vertigo/dizziness attack? J Stroke Cerebrovasc Dis 2016 ;25(10):2549-2552.
- Ihara M. Comment: Autosomal dominant small vessel disease due to heterozygous HTRA1 mutations. Neurology 2016;86 (21): 1972. doi: 10.1212/WNL.0000000000002711.
- Tojima M, Saito S, Yamamoto Y, Mizuno T, Ihara M, Fukuda H. CADASIL with a novel NOTCH3 Cys323Trp mutation presenting border zone infarcts: A case report and literature review. J Stroke Cerebrovasc Dis 2016 pii: S1052-3057(16)30084-2. doi: 10.1016/j.jstrokecerebrovasdis.2016.05.013.
- Hattori Y, Maki T, Saito S, Yamamoto Y, Nagatsuka K, Ihara M. Influence of low-dose aspirin on cerebral amyloid angiopathy in mice. J Alzheimers Dis 2016;52(3):1037-1045.
- Ihara M, Yamamoto Y. Emerging evidence for pathogenesis of sporadic cerebral small vessel disease. Stroke 2016;47(2):554-560. 10.1161/STROKEAHA.115.009627
- Hattori Y, Enmi J, Iguchi S, Saito S, Yamamoto Y, Tsuji M, Nagatsuka K, Kalaria RN, Iida H, Ihara M. Gradual carotid artery stenosis in mice closely replicates hypoperfusive vascular dementia in humans. J Am Heart Assoc 2016;5(2):e002757.
- Kalaria RN, Akinyemi R, Ihara M. Stroke injury, cognitive impairment and vascular dementia. Biochim Biophys Acta 2016; 1862 (5): 915-925.
- Tonomura S, Ihara M, Kawano T, Tanaka T, Okuno Y, Saito S, Friedland RP, Kuriyama N, Nomura R, Watanabe Y, Nakano K, Toyoda K, Nagatsuka K. Intracerebral hemorrhage and deep microbleeds associated with cnm-positive Streptococcus mutans; a hospital cohort study. Sci Rep 2016;6:20074. doi: 10.1038/srep20074.
- Saito S, Ihara M. Interaction between cerebrovascular disease and Alzheimer pathology. Curr Opin Psychiatry 2016;29(2):168-173. doi: 10.1097/YCO.0000000000000239.
- Kitamura A, Saito S, Maki A, Ayaki T, Oishi N, Kalaria RN, Hattori Y, Yamamoto Y, Urushitani M, Horsburgh K, Fukuyama H, Takahashi R, Ihara M. Gradual cerebral hypoperfusion in spontaneously hypertensive rats induces slowly evolving white matter abnormalities and impairs working memory. J Cereb Blood Flow Metab 2016;36(9):1592-1602.
- Chen A, Akinyemi RO, Hase Y, Firbank MJ, Ndung'u MN, Foster V, Craggs LJL, Washida K, Okamoto Y, Thomas AJ, Polvikoski TM, Allan JM, Oakley AE, O'Brien JT, Horsburgh K, Ihara M, Kalaria RN. Frontal white matter hyperintensities, clasmatodendrosis and gliovascular abnormalities in ageing and post-stroke dementia. Brain 2016;139(Pt 1):242-258.
- Kim J, Yoon H, Horie T, Burchett J, Restivo J, Rotllan N, Ramírez C, Verghese P, Ihara M, Hoe HS, Esau C, Fernández-Hernando C, Holtzman D, Cirrito J, Ono K, Kim J. microRNA-33 Regulates ApoE Lipidation and Amyloid-β Metabolism in the Brain. J Neurosci 2015;35(44):14717-14726.
- Miyamoto N, Maki T, Shindo A, Liang A, Maeda M, Egawa N, Itoh K, Lo E, Lok J, Ihara M, Arai K. Astrocytes promote oligodendrogenesis after white matter damage via brain-derived neurotrophic factor. J Neurosci 2015;35(41):14002-14008.
- Tanaka T, Yamagami H, Ihara M, Motoyama R, Fukuma K, Miyagi T, Nishimura K, Toyoda K, Nagatsuka K. Seizure outcomes and predictors of recurrent post-stroke seizure:a retrospective observational cohort study. PLOS ONE 2015;10(8):e0136200.
- Taguchi A, Sakai C, Soma T, Kasahara Y, Stern DM, Kajimoto K, Ihara M, Daimon T, Yamahara K, Doi K, Kohara N, Nishimura H, Matsuyama T, Naritomi H, Sakai N, Nagatsuka K. Intravenous Autologous Bone Marrow Mononuclear Cell Transplantation for Stroke: Phase1/2a Clinical Trial in a Homogeneous Group of Stroke Patients. Stem Cells Dev 2015;24(19):2207-2218.
- Saito S, Yamamoto Y, Ihara M. Mild cognitive impairment: at the crossroad of neurodegeneration and vascular dysfunction. Curr Alzheimer Res 2015;12(6):507-512.
- Fukuma K, Ihara M, Tanaka T, Morita Y, Toyoda K, Nagatsuka K. Intracranial cerebral artery dissection of anterior circulation as a cause of convexity subarachnoid hemorrhage. Cerebrovasc Dis 2015;40:45-51.
- Craggs LJL, Fenwick R, Oakley AE, Ihara M, Kalaria RN. Immunolocalisation of PDGFRβ and pericytes in CADASIL. Neuropath Appl Neurobiol 2015;41(4):557-570.
- Hattori Y, Enmi JI, Kitamura A, Yamamoto Y, Saito S, Takahashi S, Iguchi S, Tsuji M, Yamahara K, Nagatsuka K, Iida H, Ihara M. A novel mouse model of subcortical infarcts with dementia. J Neurosci 2015;35(9):3915-3928.
- Hattori Y, Okamoto Y, Nagatsuka K, Takahashi R, Kalaria RN, Kinoshita M, Ihara M. SIRT1 attenuates severe ischemic damage by preserving cerebral blood flow. NeuroReport 2015;26(3):113-117.
last updated : 2021/10/05
