Research Institute
Research Activities
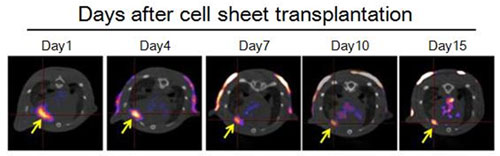
1. Development of noninvasive stem cell tracking using reporter gene imaging (Dr. Otani)
Transplantation of stem cells is a promising strategy for the treatment of ischemic diseases. However, the temporal and spatial dynamics/distribution of stem cells after transplantation has not been fully elucidated. So, we are investigating the feasibility of temporal and spatial tracking of stem cells using SPECT with human sodium/iodide symporter (hNIS) as a reporter gene.
2. Development of clinically translatable ultrasound molecular imaging (Dr. Otani)
Ultrasound molecular imaging with molecular-targeted bubbles enables the noninvasive visualization of molecular dynamics in situ. However, the clinically translatable molecular-targeted bubbles have not been developed until now. So, we are investigating the feasibility of ultrasound molecular imaging using the clinically available ultrasound contrast agent (Sonazoid).
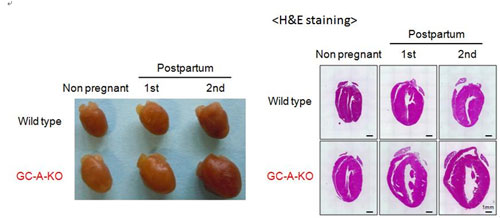
3. Clarification of the mechanisms underlying the protective role of endogenous natriuretic peptides signaling on the maternal heart during the lactation period. (Dr. Otani)
Two natriuretic peptides, atrial (ANP) and brain natriuretic peptide (BNP) act through the common receptor, guanylyl cyclase-A (GC-A) to lower blood pressure, induce diuresis/natriuresis, and dilate blood vessels (Endocr Rev 2006;27:47-72). The pivotal roles of endogenous ANP, BNP, and GC-A on the cardiovascular system have been elucidated using genetically engineered mice (FEBS J 2011;278:1830-41). Recently, we discovered that the significant cardiac hypertrophy was induced in the postpartum GC-A knockout mice, especially during the lactation period. This result implied that the endogenous natriuretic peptide signaling protects the maternal heart during the lactation period. Now, we are investigating the mechanisms underlying the protective role of endogenous natriuretic peptides signaling on the maternal heart during the lactation period.
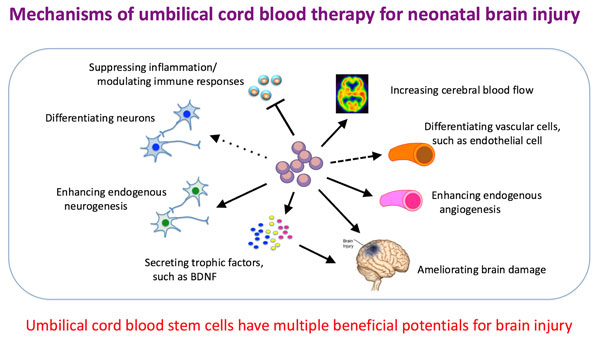
4. Preclinical study of cell-based therapies for neonatal brain damage (Drs. Tsuji, Ogawa, Ohshima, Tanaka)
Neonatal hypoxic-ischemic encephalopathy and neonatal stroke emerge unexpectedly during a perinatal period, and frequently cause life-long neurological sequelae, such as cerebral palsy and mental retardation. Using those disease models, we have been trying to develop novel cell-based therapies. We have been mainly investigating effects and mechanisms of intravenous transfusion of umbilical cord blood-derived hematopoietic stem cells. Recently, we have started studies with Muse (multilineage-differentiating stress enduring) cells and umbilical cord-derived mesenchymal stem cells with collaborations with Dr. Y. Sato at Nagoya Univ. and Prof. H. Shintaku at Osaka City Univ.
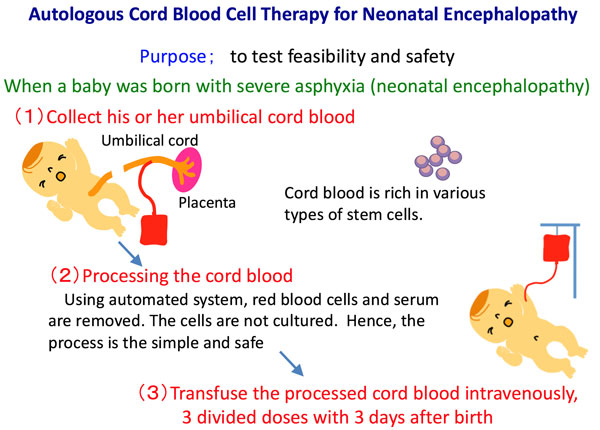
5. Clinical study of umbilical cord blood cells for neonatal brain damage (Dr. Tsuji)
We have been conducting a multicenter clinical trial of autologous cord blood cell therapy for neonatal encephalopathy (NIH ClinicalTrials.gov: NCT02256618) since 2014, as the therapy is considered to be the safest and the most feasible of all cell therapies currently being developed for neonatal brain damage.
6. Mechanisms of neonatal brain damage (Drs. Tsuji, Ogawa)
To develop novel potential therapies for neonatal brain damage, we have been investigating the mechanisms of the disease conditions, using the two models, i.e. neonatal hypoxic-ischemic encephalopathy (HI) model and neonatal stroke (middle cerebral artery occlusion, MCAO) model. Our research interest lies in cerebral blood flow and inflammation, and their association with neurological outcomes.
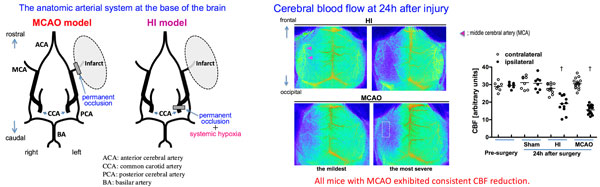
7. A novel model of prenatal brain injury (Drs. Tsuji, Ohshima, Otani)
Little progress has been made in developing effective therapies for brain injury in infants born with prematurity, compared with those born at term. One of the main reasons is lack of the model to study potential therapies. Brain injury associated with prematurity frequently leads to not only cerebral palsy but also neourodevelopmental disorders such as attention deficit/hyperactivity disorder (ADHD) and learning disorder. With collaboration with French National Centre for Scientific Research (CNRS), we are developing a model for intra-uterine hypoperfusion, which is a main cause of prenatal brain injury associated with prematurity.
8. Pharmacological therapy for cognitive dysfunction in children with Down syndrome (Drs. Tsuji, Ihara, Saito, Yamamoto, Nakaoku)
We have been investigating a potential drug to ameliorate cognitive dysfunction of Down syndrome in a mouse model of Down syndrome. The drug is already in clinical use for other purpose, hence its early translation into practice is expected once the efficacy and the safety is proved in our preclinical study.
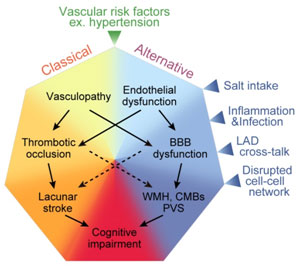
9. Generation of animal models of sporadic vascular dementia (Drs. Ihara, Hattori, Saito, Yamamoto)
We are developing animal models of sporadic vascular dementia using mice, rats, and non-human primates which can be applied to drug development for human vascular dementia. Recently, we have established a mouse model of vascular dementia named ACAS (asymmetric common carotid artery surgery) model. For this publication, our post-doc Dr. Yorito Hattori won the Kusano prize in 2016. Our review paper about cerebral small vessel disease was published in Stroke and one of the figures was printed in cover page (see figure below).
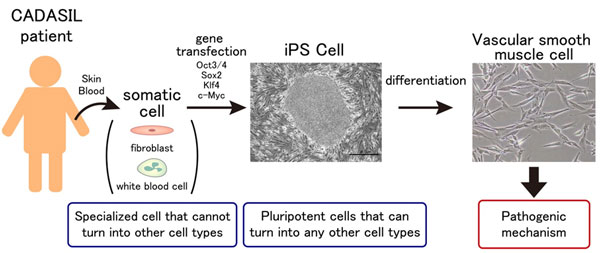
10. Research on the pathogenesis of hereditary vascular dementia CADASIL and CARASIL (Drs. Ihara, Yamamoto, Saito)
CADASIL (cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy) and CARASIL (cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy) are hereditary small vessel disease caused by mutations in NOTCH3 and HTRA1, respectively. Although the causative genes have been identified, their molecular pathogenesis is still unclear. We are investigating the pathogenesis of hereditary vascular dementia using animal models of CADASIL and CARASIL as well as induced pluripotent stem cells (iPSCs) from CADASIL patients. Currently we are also working on the establishment of CADASIL patient database. We aim to expand our research further to establish Japanese diagnostic criteria of CADASIL and develop new treatment in the future. (CADASIL Research Group: http://square.umin.ac.jp/cadasil/)
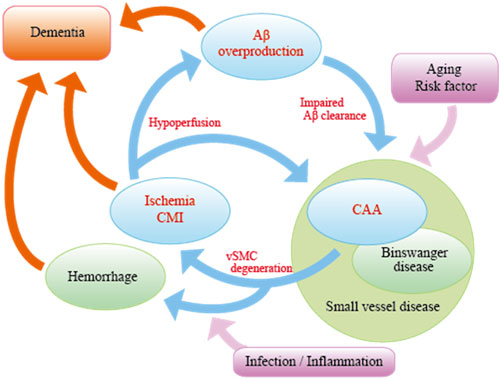
11. Vascular factors in Alzheimer's disease (Drs. Ihara, Nakaoku, Saito)
The elderly people often have multiple comorbidities. The most common cause of dementia, Alzheimer's disease, is no exception; it is accompanied by lifestyle-related disorders such as hypertension and diabetes mellitus. We therefore try to elucidate the underlying mechanism by which vascular disease accelerates neurodegenerative processes and to find treatment of Alzheimer's disease by targeting vascular diseases.
Studies of transgenic mice expressing tau gene mutations in a familial form of tauopathy (FTDP-17) implicate pathological tau in mechanisms of neurodegenerative dementia. In case of AD, however, the relationship between Aβ and tau has not been clearly elucidated. We are investigating the mechanism from both point of view; Aβ and tau.
12. Exploration of novel treatment of cerebrovascular disorders (Drs. Ihara, Tsuji, Saito, Yamamoto)
We use anti-platelet drugs for prevention of cerebral infarction but new treatment with less side effects are required because of hemorrhagic complications. We are developing such new treatments such as peptide hormone, sirtuin activators, and regenerative medicine.
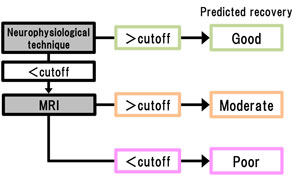
13. A study to establish an algorithm for predicting recovery after stroke (Drs. Ihara, Takahashi)
The aim of this study is to establish a new algorithm for predicting recovery after stroke in combination of neurophysiological techniques and MRI in the acute phase. By insertion of this algorithm into the inclusion criteria of future clinical trials, we aim to establish effective neuroprotective drugs.
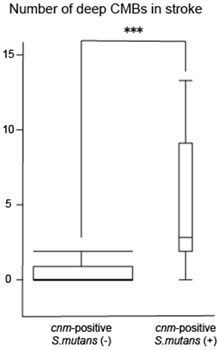
14. Research on etiology of stroke (especially cerebral hemorrhage) (Drs. Ihara, Tonomura, Saito)
A collaborative work with Osaka University Graduate School of Dentistry showed that Streptococcus mutans is a strong risk factor of cerebral hemorrhage. Although reduced in incidence due to better control of hypertension, cerebral hemorrhage is still prevalent in Japan compared to western countries, and we hope this novel finding will lead to development of new treatment of cerebral hemorrhage.
15. Development of PET/SPECT tracers for Alzheimer's disease (Dr. Ihara)
A collaborative work with Kyoto Univ Graduate School of Pharmacology and Department of Pathology of our institute aims to develop novel PET/SPECT tracers to detect pathological changes observed in Alzheimer's disease noninvasively.
16. Ongoing other projects (Dr. Ihara)
- Development of registry for post-stroke epilepsy (funded by AMED)
- Amyloid deposition & neurodegeneration in elderly Japanese with normal cognition (funded by NIH)
- Study to reduce incidence of dementia with strict control of vascular risk factors in Amagasaki City, Hyogo (supported by Amagasaki City)
- Investigator-initiated clinical trial for MCI with cilostazol: COMCID study
https://clinicaltrials.gov/ct2/show/NCT02491268
last updated:2022/06/27
