Research Institute
NTTR-NCVC Bio Digital Twin Center
Member
| Collaboration company | NTT Research, Inc. |
|---|---|
| Specially appointed director | Keita Saku, MD, PhD. |
| Laboratory chief | Kazunori Uemura, MD, PhD. |

NTTR-NCVC Bio Digital Twin Center
NTT Research (NTTR) and the National Cerebral and Cardiovascular Center (NCVC) have joined together to form a unique collaboration laboratory, the NTTR-NCVC Bio Digital Twin Center (和名:バイオデジタルツイン研究部). On April 1st, 2023, the collaboration research department was launched with the aim of creating next generation medical and healthcare applications that doctors and patients can use. This will be achieved by integrating the cardiovascular mathematical model development and validation research done at NCVC with a Bio Digital Twin (BioDT) platform promoted by NTTR.

Autonomous closed-loop intervention system
https://vimeo.com/897450724/a410ec56fb
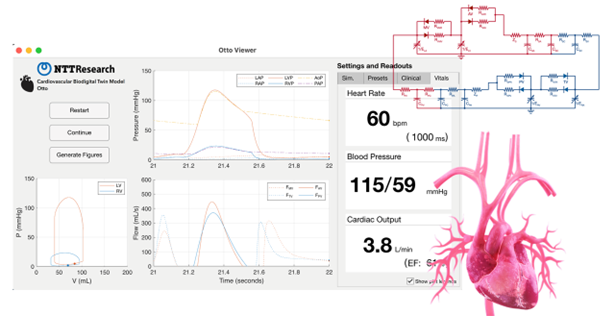
Cardiovascular simulator (Otto): Basic of Bio Digital Twin (BioDT)
https://vimeo.com/911263900/ecbc7b1435?share=copy
NTTR’s Medical and Health Informatics (MEI) lab (Director: Joe Alexander, Jr., M.D., Ph.D., F.A.C.C.) has embarked on a BioDT initiative that seeks to address the increasing complexity of underlying diseases by making use of a collection of high-fidelity artificial intelligence (AI) and physiologically-based computational models (https://ntt-research.com/mei/). As an initial project, NTTR has been developing a Cardiovascular (CV) BioDT using a class of mathematical hemodynamic models of the CV system.
NCVC also has hemodynamic mathematical models of the CV system. Moreover, NCVC has world-leading knowledge of CV regulation and advanced technologies relating to closed-loop automated therapeutic interventions for major CV diseases such as acute myocardial infarct, heart failure, and mechanical circulatory support. Furthermore, NCVC has a wet lab facility using large animals for hypothesis generation, validation, and preclinical studies for proof of concept (POC).
NCVC and NTTR have worked on a 2½ year joint research project focused on modeling & simulation, as well as on validation studies in animals since 2020. While modeling, simulation, and animal validation studies will be ongoing, it is now time to prepare to move toward clinical translation of results to human subjects.
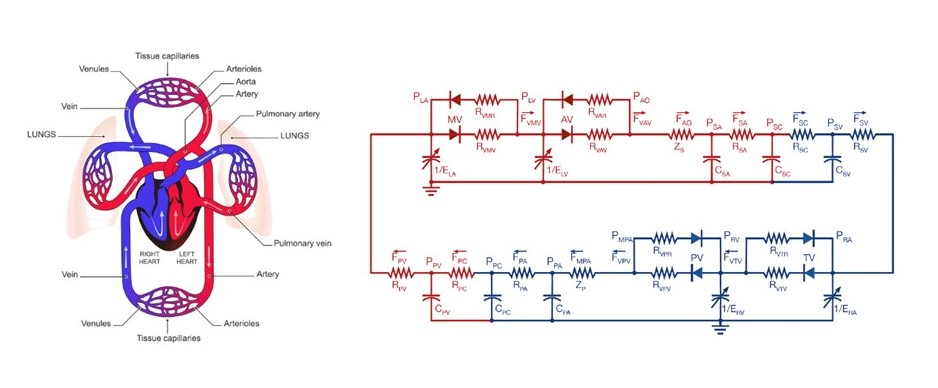
Based on these considerations, we established the collaboration research department. In the first 3 years, we will advance the following projects:
- Develop the CV BioDT platform
- Explore closed-loop therapeutic intervention and implement it into the platform of the CV BioDT
- Establish autonomous therapeutic interventions which will make the CV BioDT strategy unique and competitive.
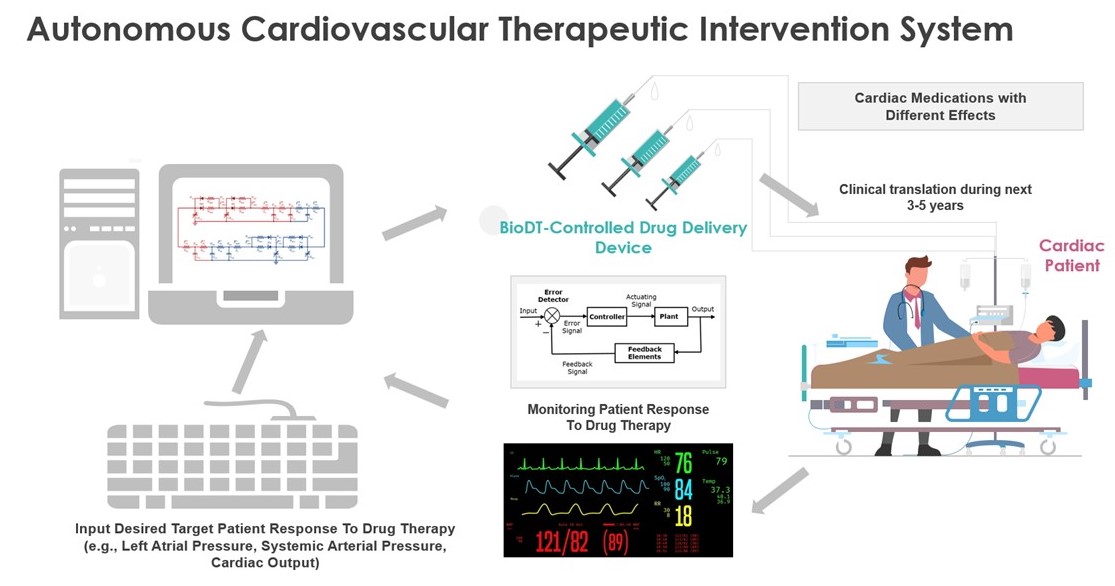
References / Publication
- NTTR video-library: https://ntt-research.com/video-library/
- Press release of collaboration research: https://www.ncvc.go.jp/topics/20210209/
- Nikkei Shinbun: https://www.nikkei.com/article/DGXZQOUF2773T0X21C22A0000000/
- Kawada T, Yamamoto H, Yokoi A, Nishiura A, Kakuuchi M, Yokota S, Matsushita H, Alexander J, Saku K. Acute effects of empagliflozin on open-loop baroreflex function and urine glucose excretion in Goto-Kakizaki diabetic rats. Journal of Physiological Sciences. J Physiol Sci. 2023;73(1):7. doi: 10.1186/s12576-023-00861-9.
- Nishikawa T, Uemura K, Hayama Y, Kawada T, Saku K, Sugimachi M. Development of an automated closed-loop β-blocker delivery system to stably reduce myocardial oxygen consumption without inducing circulatory collapse in a canine heart failure model: a proof of concept study. J Clin Monit Comput. 2022 Jun;36(3):849-860.
- Yoshida K, Saku K, Jan Bogaard H, Abe K, Sunagawa K, Tsutsui H. Vagal nerve stimulation preserves right ventricular function in a rat model of right ventricular pressure overload. Pulm Circ. 1;12(4): e12154, 2022.
- Saku K, Yokota S, Nishikawa T, Kinugawa K. Interventional heart failure therapy: A new concept fighting against heart failure. J Cardiol. 16:S0914-5087(21)00339-7,2021.
- Saku K, Nakata J. How Should We Develop New Risk Scores for Cardiogenic Shock? Circ J. Vol. 86 , No. 4 pp. 695-698, 2021.
- Uemura K, Kawada T, Zheng C, Li M, Sugimachi M. Low-Dose Landiolol Reduces Heart Rate and Cardiac Oxygen Consumption Without Compromising Initial Hemodynamic Resuscitation in a Canine Model of Endotoxin Shock. Shock. 52: 102-110, 2019.
- Uemura K, Kawada T, Zheng C, Li M, Sugimachi M. Computer-controlled closed-loop drug infusion system for automated hemodynamic resuscitation in endotoxin-induced shock. BMC Anesthesiol. 17: 145, 2017.
- Uemura K, Sugimachi M. Automated Cardiovascular Drug Infusion System to Control Hemodynamics. Adv Biomed Eng. 2: pp. 32-7, 2013.
- Uemura K, Sunagawa K, Sugimachi M. Computationally managed bradycardia improved cardiac energetics while restoring normal hemodynamics in heart failure. Ann Biomed Eng. 37: 82-93, 2009.
last updated : 2024/02/13
