Research Institute
Development of Neural Recording, Telemetry and Microdialysis Approaches for Integrated Analysis of the Pathophysiological Mechanisms in Cardiovascular Diseases
In this field we are utilizing small animal models for in vivo experiments that primarily investigate the role of endogenous neural and hormonal factors, and exogenous application of peptides in ischemic disease, hypertension and diabetes, which are increasingly becoming more prevalent in society. Here we are investigating the effects of such factors on circulatory, respiratory and metabolic dysfunction. Further, we are also investigating the potential benefits of therapies to prevent or reverse the development of these disease states.
This research is comprised of 3 sub-themes.
1) Research on Neural and Hormonal Regulation of the Circulation, Respiration and Metabolism in Freely Moving and Anaesthetised Small Animal Models.
We have developed techniques for chronic recording (one month) of blood flow, blood pressure and nerve activity from renal and splanchic nerves in conscious and freely moving rats. With these techniques we are highlighting the roles of neural regulation in the progression of hypertension and heart failure, with the aim of developing therapies that target the autonomic nervous system.
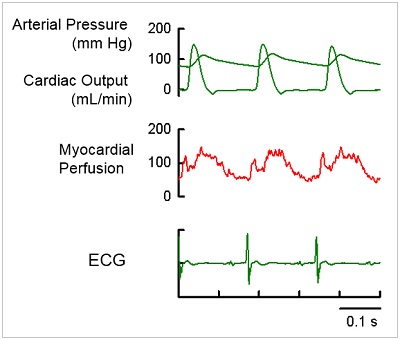
Recently, we have started experiments that enable us to record myocardial perfusion (Figure 5 & 6) in freely moving mice and rats through chronically implanted laser Doppler and fibre optic blood flow probes. This approach now makes it possible to continuously record regional blood flow in a specific region of the heart where the probe is attached in conscious and freely moving animals. In doing so, it is not only possible to make basic studies of the mechanisms of myocardial perfusion regulation, but also to monitor perfusion changes following surgical interventions, and consider the possibility of assessment of the efficacy of regenerative therapies in the ischemic heart.
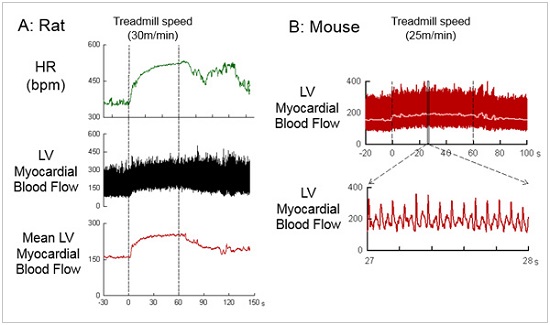
A: Heart rate (HR, bpm) and instantaneous myocardial blood flow response in a rat during treadmill running (30m/min for 60 s). Myocardial perfusion increased at the start of exercise and remained at a stable high for 30 s after exercise halted.
B: Instantaneous myocardial blood flow response during treadmill running (25m/min for 60 s) in a mouse. A double peak in blood flow in each heart beat was evident, as signal noise during activity was very low. (Dr Hirotsugu Tsuchimochi).
We are investigating in an integrative manner the molecular mechanisms concerning the coordination of circulatory, respiratory and metabolic functions on a beat by beat basis over the diurnal cycle utilizing a recording-analysis system that is able to record blood pressure, heart rate, minute volume, respiration frequency, oxygen consumption and carbon dioxide at the same time in conscious transgenic mice. More recently, we have been able to even record these physiological variables in mice during spontaneous exercise through improvements in this system.
In anaesthetized animals, we have now succeeded for the first time in recording sympathetic nerve activity specific to the pulmonary circulation. Using this method, we have been able to show the importance of neural dilatory mechanisms mediated via the β-adrenoceptor in the lung in the maintenance of global systemic circulation during hypoxic exposure. Again in anaesthetized rats during acute myocardial infarction we have found that subcutaneous administration of ghrelin prominently suppresses the dramatic rise in cardiac sympathetic nerve activity and occurrence of lethal arrhythmias. Further, in the chronic hypoxia rat model of pulmonary hypertension we have shown that ghrelin treatment is effective in suppressing lung expression of endothelin-1 and pulmonary remodeling and improving pulmonary endothelial function.
Related Publications
- Yamakoshi K., K. Yagishita, H. Tsuchimochi, T. Inagaki, M. Shirai, D.C. Poole, Y. Kano. Microvascular oxygen partial pressure during hyperbaric oxygen in diabetic rat skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 309:R1512-1520, 2015.
- Kidoya H., H. Naito, F. Muramatsu, D. Yamakawa, W. Jia, M. Ikawa, T. Sonobe, H. Tsuchimochi, M. Shirai, R.H. Adams, A. Fukamizu, N. Takakura. APJ Regulates Parallel Alignment of Arteries and Veins in the Skin. Dev Cell.33:247-259, 2015.
- Zohdi, V., J.T. Pearson, M. Kett, P. Lombardo, M. Schneider-Kolsky, and M.J. Black. When early life growth restriction in rats is followed by attenuated postnatal growth: effects on cardiac function in adulthood. Eur J Nutr 54(5):743-750, 2015.
- Zohdi V., J. Lim, J.T. Pearson and M.J. Black. Developmental programming of cardiovascular disease following intrauterine growth restriction: findings using a rat model of maternal protein restriction. Nutrients 7:119-152, 2015.
- Ngo, J.P., S. Kar, M.M. Kett, B.S. Gardner, J.T. Pearson, D.W. Smith, J. Ludbrook, J.F. Bertram, and R.G. Evans. Vascular geometry and oxygen diffusion in the vicinity of artery-vein pairs in the kidney. Am J Physiol Renal Physiol 207: F1111-F1122, 2014.
- Yokoyama U., S. Minamisawa, A. Shioda, R. Ishiwata, M-H. Jin, M. Masuda, T. Asou, Y. Sugimoto, H. Aoki, T. Nakamura, Y. Ishikawa. Prostaglandin E2 Inhibits Elastogenesis in the Ductus Arteriosus via EP4 Signaling. Circulation 129:487-496, 2014.
- Okumura S., T. Fujita, W. Cai, M. Jin, I. Namekata, Y. Mototani, H. Jin, Y. Ohnuki, Y. Tsuneoka, R. Kurotani, K. Suita, Y. Kawakami, S. Hamaguchi, T. Abe, H. Kiyonari, T. Tsunematsu,Y. Bai, S. Suzuki, Y. Hidaka, M. Umemura, Y. Ichikawa, U. Yokoyama, M. Sato, F. Ishikawa, H. Izumi-Nakaseko, S. Adachi-Akahane, H. Tanaka and Y. Ishikawa. Epac1-dependent phospholamban phosphorylation mediates the cardiac response to stresses. Journal of Clinical Investigation 124(6):2785-2801, 2014.
- Skiold B., Q. Wu, S.B. Hooper, P.G. Davis, M. Tolcos, J. Pearson, R. Vreys, G. Egan, S.K. Barton, J.L-Y. Cheong, G.R. Polglase. Early detection of ventilation induced brain injury using magnetic resonance spectroscopy and diffusion tensor imaging: an in vivo study in preterm lambs. PLoS One 9(4):e95804, 2014.
- Nakaoka H., Y. Nakagawa-Toyama, M. Nishida, T. Okada, R. Kawase, T. Yamashita, M. Yuasa-Kawase, K. Nakatani, D. Masuda, T. Ohama, T. Sonobe, M. Shirai, I. Komuro and S. Yamashita. Establishment of a novel murine model of ischemic cardiomyopathy with multiple diffuse coronary lesions. PLoS One 8(8): e70755, 2013.
- Ogura S., T. Shimosawa, S. Mu, T. Sonobe, F. Kawakami-Mori, H. Wang, Y. Uetake, K.I. Yoshida, Y. Yatomi, M. Shirai and T. Fujita. Oxidative stress augments pulmonary hypertension in chronically hypoxic mice overexpressing oxidized LDL receptor. Am J Physiol Heart Circ Physiol 305(2): H155-H162, 2013.
- Maeda H., H. Nagai, G. Takemura, K. Shintani-Ishida, M. Komatsu, S. Ogura, T. Aki, M. Shirai, I. Kuwahira, K-I. Yoshida. Intermittent-hupoxia induced autophagy attenuates contractile dysfunction and myocardial injury in rat heart. Biochim Biophys Acta 1832(8): 1159-1166, 2013.
- Fujii Y., M. Shirai, S. Inamori, A. Shimouchi, T. Sonobe, H. Tsuchimochi, J.T. Pearson, Y. Takewa, E. Tatsumi and Y. Taenaka. Insufflation of hydrogen gas restrains the inflammatory response of cardiopulmonary bypass in a rat model. Artif Organs 37(2): 136-141, 2013.
- Inamori S., M. Shirai, N. Yahagi, J.T. Pearson, Y. Fujii, K. Umetani, Y. Kobayashi, Y. Okura, M. Yakehiro and M. Minamiyama. A comparative study of cerebral microcirculation during pulsatile and nonpulsatile selective cerebral perfusion: Assessment by synchrotron radiation microangiography. ASAIO J 59(4): 374-379, 2013.
- Fujii Y., M. Shirai, H. Tsuchimochi, J.T. Pearson, Y. Takewa, E. Tatsumi and Y. Taenaka. Hyperoxic condition promotes an inflammatory responses during cardiopulmonary bypass in a rat model. Artif Organs 37:1034-1040, 2013.
- Schwenke D.O., T. Tokudome, I. Kishimoto, T. Horio, P.A. Cragg, M. Shirai and K. Kangawa, One dose of ghrelin prevents the acute and sustained increase in cardiac sympathetic tone after myocardial infarction. Endocrinology 153(5): 2436-2443, 2012.
- Higuchi K., Y. Nakaoka, W. Shioyama, Y. Arita, T. Hashimoto, T. Yasui, K. Ikeoka, T. Kuroda, T. Minami, K. Nishida, Y. Fujio, K. Yamauchi-Takihara, M. Shirai, N. Mochizuki and Komuro I. Endothelial Gab1 deletion accelerates angiotensin II-dependent vascular inflammation and atherosclerosis in apolipoprotein E knockout mice. Circ J 76(8): 2031-2040, 2012.
- Shioyama W., Y. Nakaoka, K. Higuchi, T. Minami, Y. Taniyama, K. Nishida, H. Kidoya, T. Sonobe, H. Naito, Y. Arita, T. Hashimoto, T. Kuroda, Y. Fujio, M. Shirai, N. Takakura, R. Morishita, K. Yamaguchi-Takihara, T. Kodama, T. Hirano, N. Mochizuki and I. Komuro. Docking protein Gab1 is an essential component of postnatal angiogenesis after ischemia via HGF/c-Met signaling. Circ Res 108: 664-675, 2011.
2) Analysis of Autonomic Nervous System Control of Cardiovascular Function with Microdialysis
We have established a new, concise dialysis approach to the analysis of the autonomic nerve function in the regulation of cardiac function in small animals, from mouse to rabbit. Utilisation of our dialysis technique in combination with highly sensitive measurements of norepinephrine (NE) or acetylcholine (ACh) has provided a powerful method for detecting the amount of neurotransmitter release from the autonomic nerve endings (heart and adrenal medulla). Further, this method provides information regarding NE or ACh release and disposition under a variety of pathophysiological conditions in vivo (ischemia, hypoxia, and hypothermia). More recently, utilizing this approach we are investigating the benefits of ghrelin treatment for reducing autonomic dysfunction in chronic heart disease.
Related Publications
- Abe C., Y. Nagai, S. Shimizu, T. Akiyama, T. Kawada, M. Sugimachi and H. Morita. Reduced carotid baroreceptor distensibility-induced baroreflex resetting contributes to impairment of sodium regulation in rats fed a high-fat diet. Am J Physiol Heart Circ Physiol 308, H942-950, 2015.
- Sata Y., T. Kawada, S. Shimizu, A. Kamiya, T. Akiyama and M. Sugimachi. Predominant role of neural arc in sympathetic baroreflex resetting of spontaneously hypertensive rats. Circ J 79, 592-599, 2015.
- Shimizu S., T. Kawada, T. Akiyama, M.J. Turner, T. Shishido, A. Kamiya, M. Shirai and M. Sugimachi. Guanfacine enhances cardiac acetylcholine release with little effect on norepinephrine release in anesthetized rabbits. Auton Neurosci 187, 84-87, 2015.
- Kawada T., T. Akiyama, S. Shimizu, Y. Sata, M.J. Turner, M. Shirai and M. Sugimachi. Acute effects of arterial baroreflex on sympathetic nerve activity and plasma norepinephrine concentration. Auton Neurosci 186, 62-68, 2014.
- Du C.K., D.Y. Zhan, T. Akiyama, T. Sonobe, T. Inagaki and M. Shirai. Myocardial interstitial serotonin and its major metabolite, 5-hydroxyindole acetic acid levels determined by microdialysis technique in rat heart. Life Sci 117, 33-39, 2014.
- Shimizu S., T. Akiyama, T. Kawada, A. Kamiya, M.J. Turner, H. Yamamoto, T. Shishido, M. Shirai and M. Sugimachi. Medetomidine suppresses cardiac and gastric sympathetic nerve activities but selectively activates cardiac vagus nerve. Circ J 78, 1405-1413, 2014.
- Komaki F., T. Akiyama, T. Yamazaki, H. Kitagawa, S. Nosaka and M. Shirai. Effects of intravenous magnesium infusion on in vivo release of acetylcholine and catecholamine in rat adrenal medulla. Auton Neurosci 177, 123-128, 2013.
- Kawada T., T. Akiyama, S. Shimizu, A. Kamiya, K. Uemura, M.J. Turner, M. Shirai and M. Sugimachi. Sympathetic afferent stimulation inhibits central vagal activation induced by intravenous medetomidine in rats. Acta Physiol 209, 55-61, 2013.
- Zhan D.Y., C.K. Du, T. Akiyama, T. Sonobe, H. Tsuchimochi, S. Shimizu, T. Kawada and M. Shirai. In vivo monitoring of acetylcholine release from cardiac vagal nerve endings in anesthetized mice. Auton Neurosci 176: 91-94, 2013.
- Sonobe T., T. Akiyama, C.K. Du, D.Y. Zhan and M. Shirai. Contribution of serotonin uptake and degradation to myocardial interstitial serotonin levels during ischemia-reperfusion in rabbits. Acta Physiol 207: 260-268, 2013.
- Kawada T., T. Akiyama, S. Shimizu, A. Kamiya, K. Uemura, Y. Sata, M. Shirai and M. Sugimachi. Central vagal activation by ?2-adrenergic stimulation is impaired in spontaneously hypertensive rats. Acta Physiol 206: 72-70, 2012.
- Shimizu S., T. Akiyama, T. Kawada, Y. Sata, M. Mizuno, A. Kamiya, T. Shishido, T. Inagaki, M. Shirai, S. Sano and M. Sugimachi. Medetomidine, an ?2-adrenergic agonist, activates cardiac vagal nerve through modulation of baroreflex control. Circulation Journal 76: 152-159, 2012.
- Shimizu S., T. Akiyama, T. Kawada, T. Sonobe, A. Kamiya, T. Shishido, T. Tokudome, H. Hosoda, M. Shirai, K. Kangawa and M. Sugimachi. Centrally administered ghrelin activates cardiac vagal nerve in anesthetized rabbits. Auton Neurosci 162: 60-65, 2011.
3) Relationships Between Skeletal Muscle Contractions and Circulatory Control: Research on the Mechanism of Abnormal Blood Pressure Increases During Exercise in Peripheral Arterial Disease.
In recent years there has been an increase in the prevalence of peripheral arterial disease (PAD) due to the aging of the Japanese population and change in lifestyle and increased consumption of Western style foods. Symptoms that are often reported by patients with lower limb PAD are that even if they walk a short distance the lower limb hurts, if they stop walking the pain disappears, and after resting a while they are able to continue walking again. These are the symptoms of so-called claudicatio intermittens (intermittent lameness), where it has been reported that despite the low intensity of exercise effort, blood pressure increases abnormally as a result of this walking effort. To deepen our understanding, we investigated the blood pressure responses in an animal model of PAD during static muscle contraction. We found that contractions in hypoperfused muscle fibres caused abnormal excitation in the muscle primary afferents, particularly in the group III and IV fine nerve fibres, and this elicited an abnormal increase sympathetic nerve activity, resulting in a rise in blood pressure.
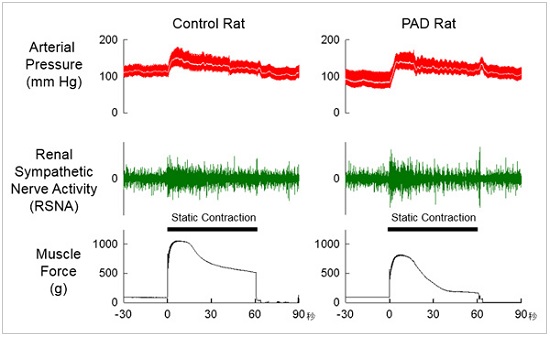
We reported the cause of that abnormal excitation is most likely the result of activation of acid sensitive ion channels (ASIC) and ATP channels expressed on group III and group IV nerve fibres following a decrease in local pH as a result of the increase in metabolic biproducts.
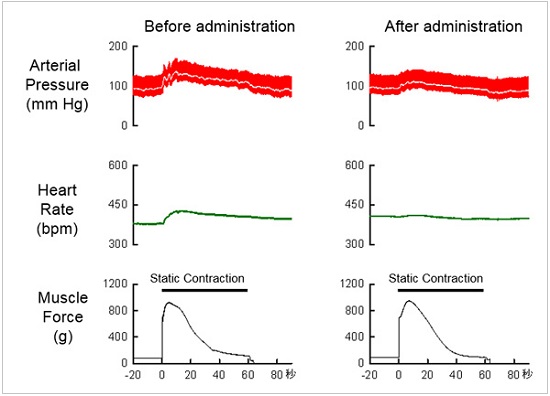
We also found that selective stimulation of opioid receptors expressed on peripheral afferent fibres appear to suppress the abnormal increase in blood pressure in PAD during skeletal muscle contraction through a potent suppressive effect of central opioid receptor signaling. Hereafter, we will attempt to shed new light on the abnormal neural mediated regulation of the circulation in PAD from both afferent and efferent perspectives by recording afferent nerve activity from the various organs together with the aforementioned efferent autonomic nerve activity.
Related Publications
- Yamauchi K., H. Tsuchimochi, A.J. Stone, S.D. Stocker and M.P. Kaufman. Increased dietary salt intake enhances the exercise pressor reflex. Am J Physiol Heart Circ Physiol. 306:H450-454, 2014.
- Tsuchimochi H., K. Yamauchi, J.L. McCord and M.P. Kaufman. Blockade of acid sensing ion channels attenuates the augmented exercise pressor reflex in rats with chronic femoral artery occlusion. The Journal of Physiology. 589: 6173-6189, 2011.
- Leal A.K., J.L. McCord, H. Tsuchimochi and M.P. Kaufman. Blockade of the TP receptor attenuates the exercise pressor reflex in decerebrated rats with chronic femoral artery occlusion. American Journal of Physiology-Heart and Circulatory Physiology. 301 (5): H2140-2146, 2011.
- McCord J.L., H. Tsuchimochi, K. Yamauchi, A.K. Leal and M.P. Kaufman. Tempol attenuates the exercise pressor reflex independently of neutralizing reactive oxygen species in femoral arterial ligated rats. Journal of Applied Physiology. 111: 971-979, 2011.
- Tsuchimochi H., J.L. McCord, A.K. Leal and M.P. Kaufman. Dorsal root tetrodotoxin-resistant sodium channels do not contribute to the augmented exercise pressor reflex in rats with chronic femoral artery occlusion. American Journal of Physiology-Heart and Circulatory Physiology. 300(2): H652-663, 2011.
- McCord J.L., H. Tsuchimochi and M.P. Kaufman. P2X2/3 and P2X3 receptors contribute to the metaboreceptor component of the exercise pressor reflex in decerebrated cats. Journal of Applied Physiology. 109(5): 1416-1423, 2010.
- Tsuchimochi H., J.L. McCord and M.P. Kaufman. Peripheral μ-opioid receptors attenuate the augmented exercise pressor reflex in rats with chronic femoral artery occlusion. American Journal of Physiology-Heart and Circulatory Physiology. 299(2): H557-565, 2010.
- Tsuchimochi H., J.L. McCord and M.P. Kaufman. Chronic femoral artery occlusion augments exercise pressor reflex in decerebrated rats. American Journal of Physiology-Heart and Circulatory Physiology. 299(1): H106-113, 2010.
last updated:2021/10/01
