Research Institute
Basic Research in Heart Failure Mechanisms, Regenerative Medicine and Therapeutics
1) Heart Failure Mechanisms and Regenerative Medicine Research
This research aims to advance our understanding of the initial root causes of the relaxation and contractile dysfunction of the left ventricle (LV) that occurs in heart failure (HF) at both the micro- and macro-levels. This research also aims to translate these findings into new therapies for HF. The first unique characteristic of this research that we have demonstrated is our experience in utilising an X-ray radiation diffraction method with high intensity synchrotron radiation to evaluate contractile protein dynamic actions in different regions of the heart simultaneous with LV pressure-volume measurements to understand the main mechanisms involved in HF and diabetic heart disease. Second, we are able to investigate HF mechanisms in situ with world leading expertise in microangiography, microdialysis and neural recordings to determine the integrated contributions of coronary microcirculation dysfunction, renal and autonomic neural dysfunctions.
Recent reports indicate that more than 50% of patients diagnosed with HF are classified as HF with preserved ejection fraction (HFpEF). The combination of diabetes with other risk factors including ageing, atrial fibrillation, hypertension and obesity often leads to HFpEF. The main factors that contribute to adverse changes in cardiac contractile protein changes are long-term insulin resistance, inflammation and increased release of catecholamines. On the basis of our recent results, as it is now recognised that cytokines, protein kinases and oxidative stress generated from immune cells, and or post-translation modifications of contractile proteins (e.g. phosphorylation, nitrosylation) potentiate early cardiac muscle, endothelial and smooth muscle cell dysfunction in HF it is important to investigate the effectiveness of selective inhibitors of these pathways. From this synchrotron research we now understand that PKCβ and Rho-kinase (ROCK) inhibitors are potential candidates for early intervention in HFpEF [see section 2 below].
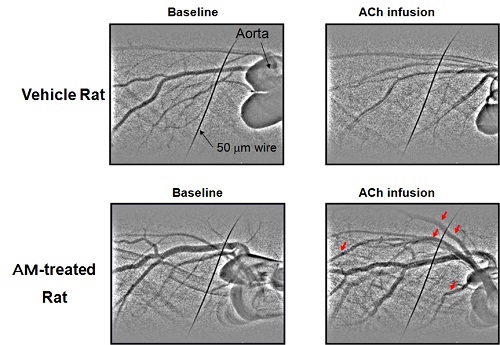
To date we have assessed various methods of regeneration therapy in models of HF with reduced ejection fraction (HFrEF), such as permanent myocardial infarction and ischemia-reperfusion injury. Utilising a custom X-ray lab source and microfocus imaging system in the Department we routinely image the vasculature in small animal models and vascular casts of organ samples, and at the SPring-8 synchrotron facility perform high resolution microangiography studies and cardiac X-ray diffraction studies on anaesthetized animals to investigate the efficacy of novel therapies. Using these approaches with a rat model of chronic heart failure (myocardial infarction) we showed that contractions of an iPS cell sheet transplanted on the infarct region were synchronous with the remote myocardium (Collaboration with Osaka University) (Figure 1). Also we have assessed the regeneration of the microcirculation from cell sheets incorporating iPS cells and myoblasts (Collaboration with Osaka University), as well as peptides such as adrenomedullin and ghrelin, and adenosine receptor ligands. Currently, in collaboration with Kyoto University Graduate School and Kyoto University Center for iPS Cell Research and Application (CiRA), we are advancing new research into iPS cell sheet therapy in ischemic cardiomyopathy hamsters.
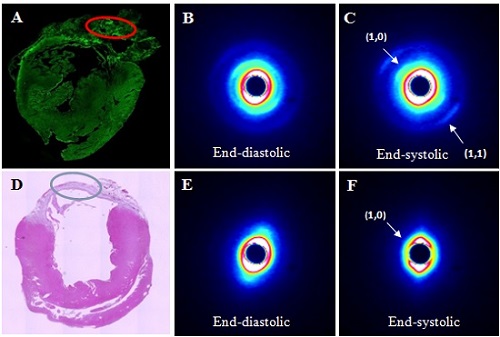
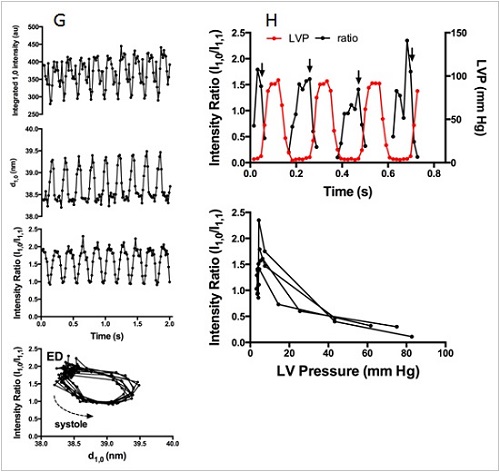
Further, utilising our expertise in microdialysis we are shedding new light on the mechanisms of myocardial dysfunction in ischemia reperfusion injury. In the future, with this technique we will now also focus on the role of catecholamines and hormones in the initiation and progression of HFpEF. In collaboration with Otago University microRNA will used to mine cardiac muscle samples for early markers of HFpEF. Lastly, we will shortly incorporate large animal models of HF in this line of research at the Australian Synchrotron and adjacent imaging facilities at Monash University where large animal imaging is possible, allowing us to evaluate coronary microcirculatory, renal and autonomic neural roles in contractile function in HFpEF through international collaboration (Australia-New Zealand-Japan).
Related Publications
- Furtado, M.B., J.C. Wilmans, A. Chandran, M. Tonta, C. Biben, M. Eichenlaub, H.A. Coleman, S. Berger, R. Bouveret, R. Singh, R.P. Harvey, M. Ramialison, J.T. Pearson, H. Parkington, N.A. Rosenthal, M.W. Costa. A novel mouse model for the study of Nkx2-5 reveals transcriptional control of cardiac ion channels. Differentiation 91:29-41, 2016.
- Katare R., S. Rawal, P.E. Munasinghe, H. Tsuchimochi, T. Inagaki, Y. Fujii, P. Dixit, K. Umetani, K. Kangawa, M. Shirai, and D.O. Schwenke. Ghrelin promotes functional angiogenesis in a mouse model of critical limb ischemia through activation of proangiogenic microRNAs. Endocrinology 157(2):432-445, 2016.
- Kainuma, S., S. Miyagawa, S. Fukushima, J. Pearson, Y-C. Chen, A. Harada, H. Watabe, G. Horitsugi, M. Ishibashi, H. Ikeda, H. Tsuchimochi, T. Sonobe, Y. Fujii, H. Naito, K. Umetani, T. Shimizu, T. Okano, E. Kobayashi, T. Daimon, A. Saito, T. Ueno, T. Kuratani, K. Toda, N. Takakura, J. Hatazawa, M. Shirai, Y. Sawa. Cell-sheet therapy with omentopexy promotes arteriogenesis and improves coronary circulation physiology in failing heart. Molecular Therapy 23(2):374-86, 2015.
- Higuchi T., S. Miyagawa, J.T. Pearson, S. Fukushima, A. Saito, H. Tsuchimochi, T. Sonobe, Y. Fujii, N. Yagi, A. Alberto, M. Shirai, Y. Sawa. Functional and electrical integration of induced pluripotent stem cell-derived cardiomyocytes in an acute myocardial infarction rat model. Cell Transplantation.24:2479-2489, 2015.
- Shirai M., N. Joe, H. Tsuchimochi, T. Sonobe, D.O. Schwenke. Ghrelin Supresses Sympathetic Hyperexcitation in Acute Heart Failure in Male Rats: Assessing Centrally and Peripherally Mediated Pathways. Endocrinology. 156:3309-3316, 2015.
- Jiao Q., Q. Ke, W. Li, M. Jin, Y. Luo, L. Zhang, D. Yang and X. Zhang. Effect of inflammatory factor-induced cyclo-oxygenase expression on the development of reperfusion-related no-reflow phenomenon in acute myocardial infarction. Clinical and Experimental Pharmacology and Physiology 42, 162-170, 2015.
- Larsimont J.C., K.K.Youssef, A. Sanchez-Danes, V. Sukumaran, M. Defrance, B. Delatte, M. Liagre, P. Baatsen, J.C. Marine, S. Lippens, C. Guerin, V. Del Marmol, J.M. Vanderwinden, F. Fuks and C. Blanpain. Sox9 Controls Self-Renewal of Oncogene Targeted Cells and Links Tumor Initiation and Invasion. Cell Stem Cell. 17(1): 60-73, 2015.
- Furtado M.B., M.W. Costa, E.M. Adi Pranoto, E. Salimova, A.R. Pinto, N.T. Lam, A. Park, P. Snider, A. Chandran, R.P. Harvey, R. Boyd, S.J. Conway, J.T. Pearson, D.M. Kaye, N. Rosenthal. Cardiogenic genes expressed in cardiac fibroblasts contribute to heart development and repair. Circ Res 114:1422-1434, 2014.
- Sonobe T., T. Akiyama, C.K. Du, D.Y. Zhan, M. Shirai. Contribution of calpain to myoglobin efflux from cardiomyocyte during ischemia and after reperfusion in anesthetized rat. Acta Physiol 210, 823-831, 2014.
- Du C.K., D.Y. Zhan, S. Morimoto, T. Akiyama, D.O. Schwenke, H. Hosoda, K. Kangawa, M. Shirai. Survival benefit of ghrelin in the heart failure due to dilated cardiomyopathy. Pharmacol Res Perspect 5, e00064, 2014.
- Sakurai S., Y. Kuroko, S. Shimizu, T. Kawada, T. Akiyama, T. Yamazaki, M. Sugimachi, S. Sano. Effects of intravenous cariporide on release of norepinephrine and myoglobin releases during myocardial ischemia/reperfusion in rabbits. Life Sci 114, 102-106, 2014.
- Shirai, M., D.O. Schwenke, H. Tsuchimochi, N. Yagi, K. Umetani, and J.T. Pearson. Synchrotron radiation imaging for advancing our understanding of cardiovascular function. Circ Res 112:209-221, 2013.
- Shirai, M., M. Beard, J.T. Pearson, T. Sonobe, H. Tsuchimochi, Y. Fujii, E. Gray, K. Umetani, D.O. Schwenke. Impaired pulmonary blood flow distribution in congestive heart failure - assessed using synchrotron radiation microangiography. J Synchrotron Rad 20:441-448, 2013.
2) Fundamental Structural and Functional Imaging Studies of the Cardiovascular System
The heart remains one of the biggest challenges for visualizing its physiological state and effectively treating acute and chronic conditions because of its continuous dynamic state. Moreover, as in other organ systems, the microcirculation, which regulates myocardial perfusion, remains a challenge for assessment using non-invasive imaging techniques. One of the most lethal conditions, resulting from interruption of blood flow to heart muscle (ischemia) via coronary arterial system, is clinically evaluated by coronary angiography with a spatial resolution of 100-200 micrometer and is treated by bypass surgery or percutaneous transluminal coronary angioplasty (PTCA: mechanical inflation of the narrowing site by balloon). These procedures cannot be applied to small coronary vessels with a diameter of less than 200μm (Intractable ischemic disease). However, microangiography with synchrotron radiation has enabled us to visualize the microcirculation even in the spontaneously beating hearts of small animals. Further, our department has a long track record in the development of X-ray imaging with lab microfocus systems for preclinical cardiovascular imaging. These X-ray imaging approaches are used extensively by us with international collaborators to investigate the mechanisms regulating microvessel perfusion in various organs in situ (heart, lung, kidney, brain, limb) in small animal models in both healthy and disease conditions. We are now also applying these approaches to study in vivo circulations in large animal models.
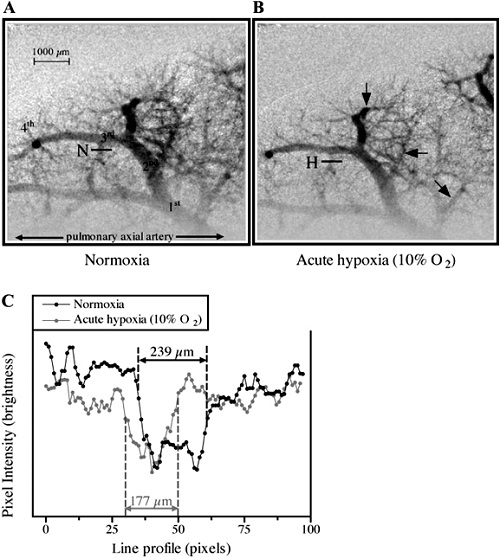
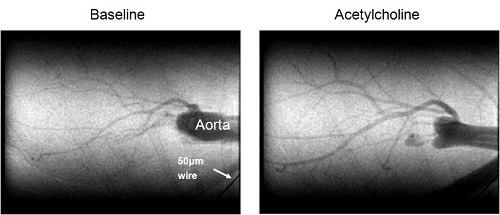
Related Publications
- Chen, Y.C., T. Inagaki, Y. Fujii, D.O. Schwenke, H. Tsuchimochi, A.J. Edgley, K. Umetani, Y. Zhang, D.J. Kelly, M. Yoshimoto, H. Nagai, R.G. Evans, I. Kuwahira, M. Shirai*, J.T. Pearson*. (2016). Chronic intermittent hypoxia accelerates coronary microcirculatory dysfunction in insulin resistant Goto-Kakizaki rats. Am J Physiol Reg Integr Comp Physiol. (accepted 31 May)
- Lang J.A.R, J.T. Pearson, C. Binder-Heschl, M.J. Wallace, M.L. Siew, M.J. Kitchen, A.B. te Pas, A. Fouras, R.A. Lewis, G.R. Polglase, M. Shirai, and S.B. Hooper. Increase in blood flow at birth; role of oxygen and lung aeration. J. Physiol. 594:1389-1398, 2016.
- Diong C., P.P. Jones, H. Tsuchimochi, E.A. Gray, G. Hughes, T. Inagaki, C.T. Bussey, Y. Fujii, K. Umetani, M. Shirai, and D.O. Schwenke. Sympathetic hyper-excitation in obesity and pulmonary hypertension: physiological relevance to the 'obesity paradox'. Int J Obes (Lond) 2016 Feb 23, doi:10.1038/ijo.2016.33
- Katare R., S. Rawal, P.E. Munasinghe, H. Tsuchimochi, T. Inagaki, Y. Fujii, P. Dixit, K. Umetani, K. Kangawa, M. Shirai, and D.O. Schwenke. Ghrelin promotes functional angiogenesis in a mouse model of critical limb ischemia through activation of proangiogenic microRNAs. Endocrinology 157(2):432-445, 2016.
- Waddingham M.T., A.J. Edgley, A. Astolfo, T. Inagaki, Y. Fujii, C-K. Du, D-Y. H. Tsuchimochi, N. Yagi, D.J. Kelly, M. Shirai and J.T. Pearson. Chronic Rho-kinase inhibition improves left ventricular contractile dysfunction in early type-1 diabetes by increasing cross-bridge myosin extension. Cardiovascular Diabetology 14:92, 2015.
- Nagai H., I. Kuwahira, D.O. Schwenke, H. Tsuchimochi, A. Nara, S. Ogura, T. Sonobe, Y. Fujii, R. Yamaguchi, L. Wingenfeld, K. Umetani, T. Shimosawa, J.T. Pearson, K. Yoshida, and M. Shirai. Activation of β3-AR/iNOS pathway in pulmonary M1 macrophage prevents development of intermittent hypoxia-induced pulmonary arterial hypertension. PLoS One 10(7):e0131923, 2015.
- Sonobe T., H. Tsuchimochi, D.O. Schwenke, and M. Shirai. Treadmill running improves hindlimb arteriolar endothelial function in type 1 diabetic mice as visualized by X-ray microangiography. Cardiovascular Diabetology 14(1):51, 2015.
- Shirai M, H. Nagai, E. Gray, J.T. Pearson, H. Tsuchimochi, T. Sonobe, S. Ogura, T. Inagaki, Y. Fujii, I. Kuwahira, Y. Yoshida, and D.O. Schwenke. Pulmonary vascular tone is dependent on the central modulation of sympathetic nerve activity following chronic intermittent hypoxia. Bas Res Cardiol, 109(5):432, 2014.
- Lang J., J.T. Pearson, A.B. te Pas, M. Wallace, M. Siew, M. Kitchen, A. Fouras, R. Lewis, K. Wheeler, G. Polglase, M. Shirai, T. Sonobe, and S.B. Hooper. Ventilation / perfusion mismatch during lung aeration at birth. J Appl Physiol 117(5):535-43, 2014.
- Nagai H., I. Kuwahira, D.O. Schwenke, H. Tsuchimochi, A. Nara, T. Inagaki, S. Ogura, Y. Fujii, K. Umetani, T. Shimosawa, K. Yoshida, J.T. Pearson, and K. Uemura, M. β2-adrenergic receptor dependent attenuation of hypoxic pulmonary vasoconstriction prevents a progression of pulmonary arterial hypertension in intermittent hypoxic rats. PLoS One 9(10):e110693, 2014.
- Shirai M., D.O. Schwenke, H. Tsuchimochi, K. Umetani, N. Yagi, J.T. Pearson. Synchrotron radiation imaging for advancing our understanding of cardiovascular function. Circ Res 112(1): 209-221, 2013.
- Joshi, M.S, P. Berger, D.M. Kaye, J.T. Pearson, J.A. Bauer, and R. Ritchie. Functional relevance of genetic variations of endothelial nitric oxide synthase (NOS3) and vascular endothelial growth factor (VEGF) in diabetic coronary microvessel dysfunction. Clin Exper Pharmacol Physiol 40:253-261, 2013.
- Jenkins M.J., J.T. Pearson, D.O. Schwenke, A.J. Edgley, T. Sonobe, Y. Fujii, H. Ishibashi-Ueda, D.J. Kelly, N. Yagi, and M. Shirai. Myosin heads are displaced from actin filaments in the in situ beating rat heart in early diabetes. Biophys J. 104(5): 1065-1072, 2013.
- Pearson J.T., M.J. Jenkins, A. Edgley, T. Sonobe, M. Joshi, M.T. Waddingham, Y. Fujii, D.O. Schwenke, H. Tsuchimochi, M. Yoshimoto, K. Umetani, D.J. Kelly, and M. Shirai. Acute Rho-kinase inhibition improves coronary dysfunction in vivo, in the early diabetic microcirculation. Cardiovasc Diabetol 12:111, 2013.
- Shirai M., M. Beard, J.T. Pearson, T. Sonobe, H. Tsuchimochi, Y. Fujii, E. Gray, K. Umetani, and D.O. Schwenke. Impaired pulmonary blood flow distribution in congestive heart failure assessed using synchrotron radiation microangiography. J Synchrotron Radiat 20(3): 441-448, 2013.
- Gray E.A., H. Tsuchimochi, J.T. Pearson, T. Sonobe, Y. Fujii, M. Yoshimoto, K. Umetani, M. Shirai, and D.O. Schwenke. Assessment of the serotonin pathway as a therapeutic target for pulmonary hypertension. J Synchrotron Radiat 20(5): 756-764, 2013.
- Jenkins M.J., A.J. Edgley, T. Sonobe, K. Umetani, D.O. Schwenke, Y. Fujii, R.D. Brown, D.J. Kelly, M. Shirai, and J.T. Pearson. Dynamic synchrotron imaging of diabetic rat coronary microcirculation in vivo. Arterioscler Thromb Vasc Biol 32(2): 370-377, 2012.
- Umetani K., J.T. Pearson, D.O. Schwenke, and M.Shirai. Development of synchrotron radiation X-ray intravital microscopy for in vivo imaging of rat heart vascular function. Conf Proc IEEE Eng Med Biol Soc 2011: 7791-7794, 2011.
- Hoshino M., K. Uesugi, J. Pearson, T. Sonobe, M. Shirai and N. Yagi. Development of an X-ray real-time stereo imaging technique using synchrotron radiation. J. Synchrotron Rad. 18: 569-574, 2011.
- Schwenke D.O., J.T. Pearson, T. Sonobe, H. Ishibashi-Ueda, A. Shimouchi, K. Kangawa, K. Umetani and M. Shirai. Role of Rho kinase signaling and endothelial dysfunction in modulating blood flow distribution in pulmonary hypertension. J. Appl. Physiol. 110: 901-908, 2011.
- Sonobe T., D.O. Schwenke, J.T. Pearson, M. Yoshimoto, Y. Fujii, K. Umetani and M. Shirai. Imaging of the closed-chest mouse pulmonary circulation using synchrotron radiation microangiography. J. Appl. Physiol. 111: 75-80, 2011.
last updated:2021/10/01
